 三甲
三甲
激素用量多大会导致股骨头坏死?:2015年:大剂量激素的使用和股骨头坏死的风险:荟萃分析和系统回顾
激素用量多大会导致股骨头坏死?:2015年:大剂量激素的使用和股骨头坏死的风险:荟萃分析和系统文献回顾
作者:Michael A Mont, Robert Pivec, Samik Banerjee, Kimona Issa, Randa K Elmallah, Lynne C Jones
作者单位: Center for Joint Preservation and Replacement, Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore, Baltimore, Maryland.
译者:陶可(北京大学人民医院骨关节科)
摘要
不同激素方案对髋关节股骨头坏死发生率的影响仍不清楚。我们进行了荟萃分析和系统文献回顾,以确定以不同平均和累积剂量和治疗持续时间服用激素的患者发生股骨头坏死,以及医学诊断是否影响股骨头坏死发生率。审查了57项研究(23561名患者)。回归分析确定了激素使用与股骨头坏死发生率之间的显着相关。激素治疗> 2 g(泼尼松等效)时,股骨头坏死发生率为6.7%。系统性红斑狼疮患者的剂量与骨坏死发生率呈正相关。每增加10 mg/d与股骨头坏死率增加3.6%相关,>20 mg/d导致更高的股骨头坏死发生率。临床医生必须警惕高糖皮质激素治疗患者的股骨头坏死,尤其是系统性红斑狼疮患者。
关键词:皮质类固醇(激素);股骨头坏死;荟萃分析;髋关节;风险因素。

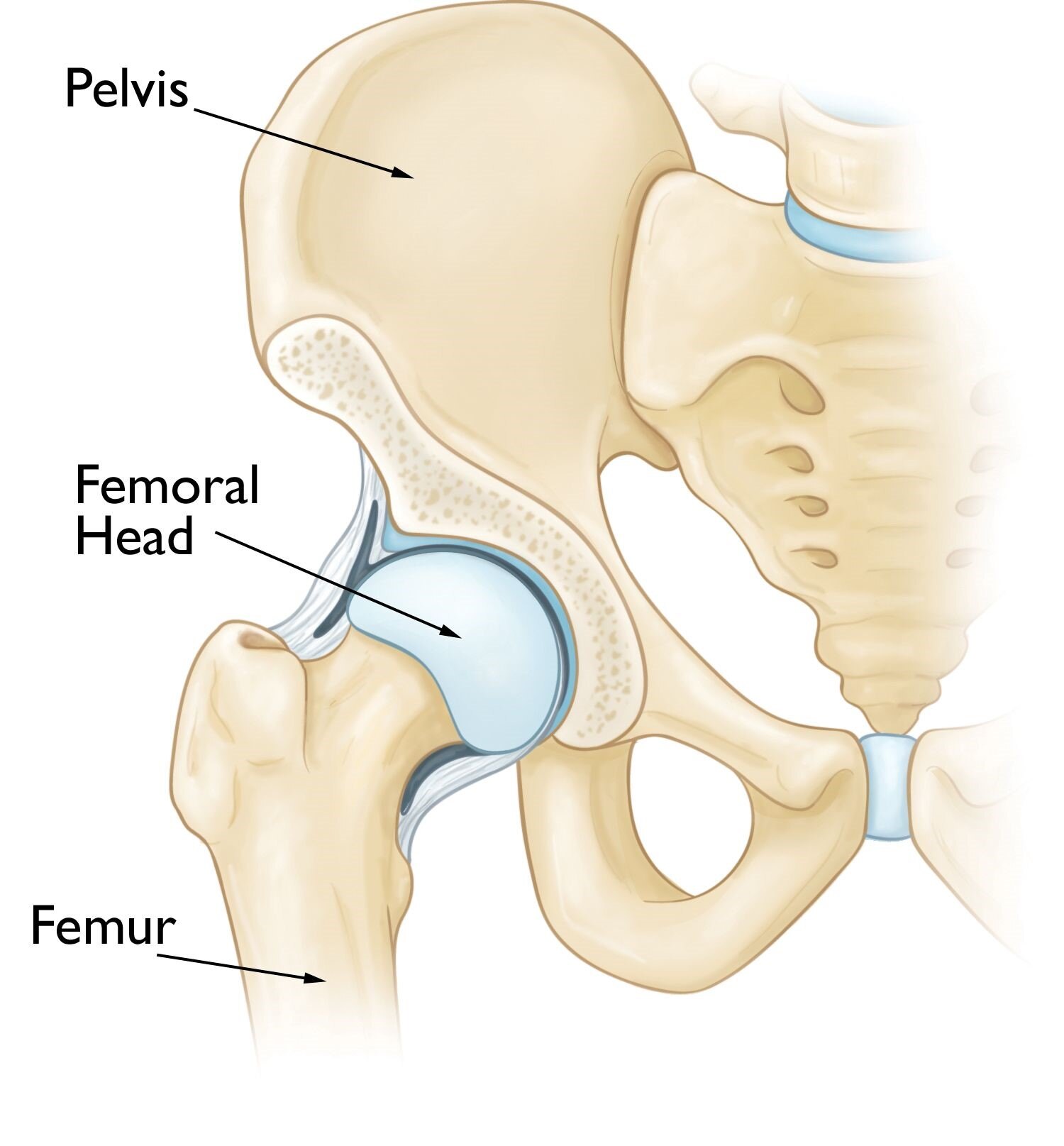
Osteonecrosis of the hip occurs in the femoral head, which is the ball of ball-and-socket hip joint.
https://orthoinfo.aaos.org/en/diseases--conditions/osteonecrosis-of-the-hip
正常髋关节结构是由球窝状的髋臼与圆球体状的股骨头形成的球窝关节。

Osteonecrosis of the Hip - OrthoInfo – AAOS
股骨头坏死是由于股骨头骨组织坏死而引发的以髋关节疼痛为临床表现的疾病。
https://orthoinfo.aaos.org/en/diseases--conditions/osteonecrosis-of-the-hip
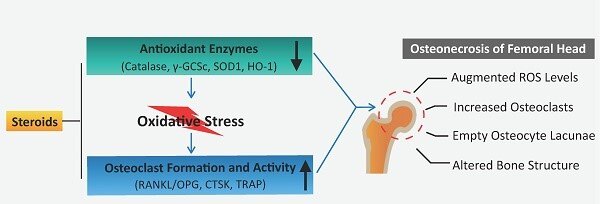
Steroid-induced osteonecrosis of the femoral head reveals enhanced reactive oxygen species and hyperactive osteoclasts: International Journal of Biological Sciences
激素诱导的股骨头坏死显示活性氧增强和破骨细胞过度活跃:国际生物科学杂志
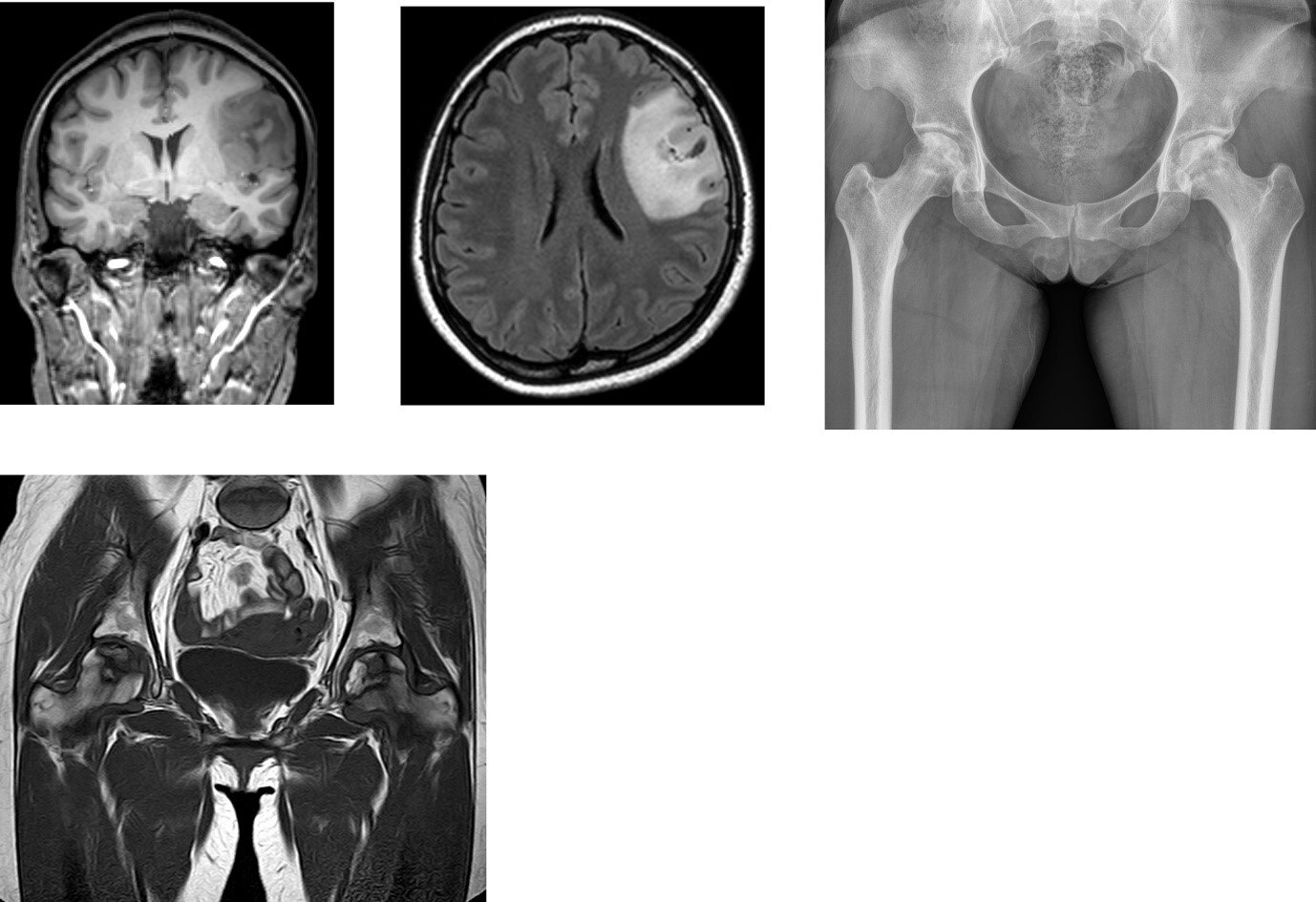
Risk factors for osteonecrosis of the femoral head in brain tumor patients receiving corticosteroid after surgery | PLOS ONE
脑肿瘤术后糖皮质激素患者股骨头坏死的危险因素 | PLOS ONE杂志

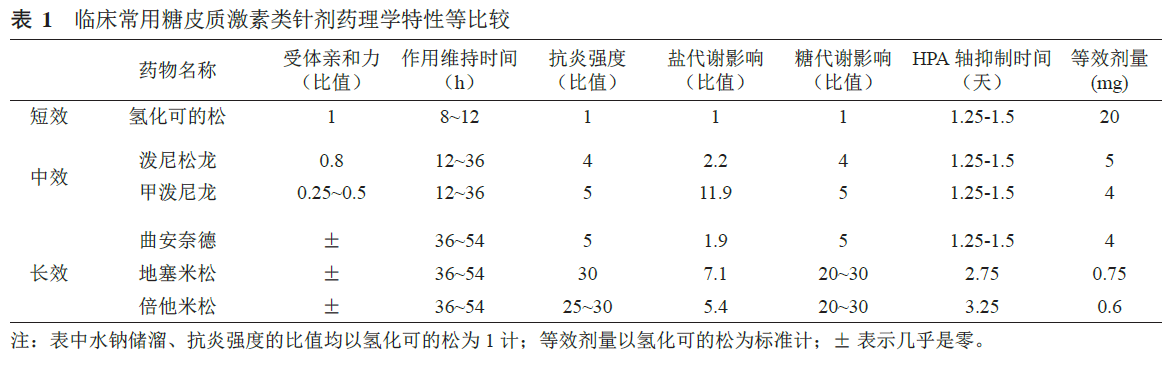
发表于《中国疼痛医学杂志》的激素换算方法:2 g波尼松龙=1.6 g甲泼尼龙=0.3 g地塞米松。
股骨头坏死可是影响髋、膝、肩和其他关节的破坏性关节疾病,并且最常见于40岁之前。在美国和欧洲,股骨头坏死占整个髋关节需要进行髋关节置换术的2%到10%,但在韩国和日本可能高达50%至60%。非创伤性股骨头坏死的病理生理机制较多,且意见尚不同意。已经报道的股骨头坏死的原因主要包括:血管损伤、异常细胞修复过程和基因点突变。而风险因素包括直接原因,如创伤、辐射暴露、血液疾病(镰状细胞病)和减压病(Caisson疾病),以及许多间接相关因素,如风湿病或代谢疾病、激素、酒精和/或吸烟。
Heimann和Freiberger是最早报告接受高剂量激素治疗的患者出现股骨头坏死病例的人之一。此后的多项研究表明,长期、高剂量激素的使用是与股骨头坏死相关的独立因素,据报道,三个月内超过2 g的剂量存在发生股骨头坏死的风险。然而,研究之间的患者人口统计学和流行病学差异存在显着的异质性。此外,很少有报告以不同医学诊断功能差异来区分股骨头坏死发生率。
本文进行了系统文献回顾和荟萃分析,以研究大剂量皮质类固醇(激素)治疗与股骨头坏死发生率的关系。主要研究问题是:(1)服用大剂量皮质类固醇(激素)的患者的总体股骨头坏死发生率是多少;(2)使用皮质类固醇(激素)的基础疾病是否会影响股骨头坏死的发生率;(3)平均剂量、累积剂量或治疗持续时间是否与发病率相关;(4)脉冲疗法是否影响发病率。
结果
股骨头坏死的发生率
系统评价表明,服用大剂量皮质类固醇(激素)的患者的总体骨坏死发生率为6.7%(范围为0.3%至52%)。两项I级研究证明累积剂量与股骨头坏死发生率之间存在显着正相关,而5项II级研究未能证明这一点。
股骨头坏死的疾病和发病率
SARS的股骨头坏死发生率为21.8%,SLE为15.7%,肾移植为14.7%,骨髓移植BMT为6.6%(图2)。在所有诊断中,我们观察到平均皮质类固醇(激素)剂量与股骨头坏死之间呈正相关(图3)。这与基础疾病无关,因为对具有不同医学诊断(SLE、严重急性呼吸综合征、骨髓移植、肾移植)的患者之间股骨头坏死发生率的方差分析表明诊断类别之间没有差异(P = 0.16)。回归分析显示SLE患者呈显着正相关(r = 0.81;R2 = 0.67;P < 0.05),但在肾移植受者中不显着(r = 0.32;R2 = 0.09;P > 0.05)。还注意到,与年龄较大的患者相比,肾移植受者和SLE患者如果年龄小于35岁,则更容易发生骨坏死(22 对 13%;P = 0.04,33 对 7%;P = 0.02,分别)。
对每天服用超过20 mg皮质类固醇(激素)的患者进行股骨头坏死的荟萃分析表明,使用皮质类固醇(激素)的患者发生股骨头坏死的几率显着高于每天服用少于20 mg的皮质类固醇(激素)(OR 9.1;95% 置信区间,4.6 至 19.8)(图4A)。对于接受高累积皮质类固醇剂量(激素)(大于10 g)治疗的患者,发生股骨头坏死的优势比为2.4(95% CI. 0.8 至 6.4),较低的给药方案与较低的股骨头坏死发生率相关(OR 0.4;95% ,置信区间 0.25 到 0.54)(图4B)。此外,我们观察到每天增加10毫克的剂量会导致骨坏死率增加3.6%。由于缺乏数据和对照组,无法进行荟萃分析比较其他给药方案或治疗持续时间对股骨头坏死风险的影响。
累积剂量和治疗持续时间与SLE和肾移植患者的发病率呈负相关。在SLE患者中,累积剂量和治疗持续时间与股骨头坏死发生率呈负相关(分别为 r = - 0.85,R2 = 0.65 和 r = - 0.53,R2 = 0.29),但均无显着性意义(P > 0.05),并且可能不代表真正的趋势。在肾移植受者中,没有证据表明累积剂量与股骨头坏死发生率之间存在显着相关性(r = 0.31;R2 = 0.42;P > 0.05)。
脉冲皮质类固醇激素治疗和股骨头坏死的发生率
在根据系统评价数据评估脉冲皮质类固醇激素效果的20项研究中,平均股骨头坏死发生率为33%。
讨论
我们的目的是评估现有文献,并利用统计方法评估皮质类固醇激素与髋关节股骨头坏死之间的关联。特别是,我们评估了给药方案和治疗持续时间的效果,以及不同疾病的作用。本研究建立在已证明股骨头坏死的独立危险因素的个别报告的基础上,这些报告在早期研究中未观察到。我们的结果表明,股骨头坏死发生率受到皮质类固醇激素治疗、皮质类固醇激素剂量、和患者年龄影响。具体而言,接受大剂量皮质类固醇激素治疗的患者发生股骨头坏死的可能性可能高达十倍,与累积剂量小于10 g相比,大于10 g的累积剂量可能会使发生股骨头坏死的可能性增加两倍。此外,在回归分析中观察到皮质类固醇激素剂量与股骨头坏死发生率之间的相关性在SLE患者中最为明显。然而,使用方差分析没有发现诊断之间的差异,需要进一步研究才能得出更强有力的结论。
多项研究表明,皮质类固醇激素是股骨头坏死的独立危险因素。Shibatani等在一项对150名患者的研究中指出,在肾移植后的头两个月内,皮质类固醇激素的总剂量与患者的股骨头坏死发生率之间存在显着关联(OR = 4,P = 0.02)[60]。Nakamura等报道了SLE患者发生股骨头坏死的几率为10.3,这与本研究的结果相似(OR = 9.1)[73]。我们还观察到平均每日皮质类固醇激素剂量、累积剂量和治疗持续时间与股骨头坏死发生率之间的强相关性(R2 > 0.8)。然而,没有单一因素可以预测股骨头坏死发生率的变异性,这表明所有三个因素之间可能存在协同效应。
潜在的诊断可能会影响发生股骨头坏死的风险。然而,尚不清楚哪种疾病起主导作用、潜在疾病或皮质类固醇激素的作用,与其他疾病相比,皮质类固醇激素可能对某些疾病具有更强的负面协同作用。Shigemura等的一项前瞻性磁共振成像(MRI)研究表明,SLE患者的股骨头坏死风险(RR 2.1)明显高于非SLE患者(37% vs 21%;P = 0.001)。然而,由于死亡率较高,他们排除了器官移植受者,并主要依赖其他全身性炎症性疾病(例如炎症性肠病、血管炎、皮肤自身免疫性疾病)作为对照组。此外,Leiberman等报道了在肝移植后平均31个月内通过MRI诊断出的股骨头坏死的发生率较低(3%)[47]。目前,潜在疾病对股骨头坏死发病率的真正影响仍有待确定,需要更多的前瞻性研究。
除了诊断上的差异外,重要的是要强调人口因素和种族对股骨头坏死发展的影响。尽管很难确定整个人群的股骨头坏死率,但一些研究评估了亚洲人群的股骨头坏死并显示出较高的疾病发病率,特别是在那些患有皮质类固醇激素依赖症的人群中。例如,Yamaguchi等证明了日本患者非创伤性股骨头核酸的发病率呈上升趋势,特别是在那些患有皮质类固醇激素依赖症的患者中,在使用大剂量皮质类固醇激素的 SLE 患者中的发病率高达44%。然而,这是一种真正的趋势,还是仅仅是诊断能力的提高,尚无定论。此外,Fukushima等观察到,根据日本特发性股骨头坏死研究委员会的数据,每年约有2200名新患者被诊断为股骨头坏死。相比之下,美国的研究表明股骨头坏死率要低得多,SLE患者的股骨头坏死患病率约为15%。已被报道基因突变的种族特异性差异,例如因子V Leiden和凝血酶原,然而,股骨头坏死发病率差异的确切原因仍不清楚,也可能是特发性的或归因于生活方式。尽管如此,仍然重要的是要强调某些患者对皮质类固醇激素的反应方式可能存在根本差异。
过去二十年发表的几项研究表明,高剂量皮质类固醇激素与股骨头坏死发生率之间存在关联,尽管其他研究没有这种趋势。与本荟萃分析中观察到的结果相似,先前的系统评价发现皮质类固醇激素剂量每天每增加10 mg,股骨头坏死率增加4.6%(图 2;本研究每10 mg/天增加10%)[67]。此外,平均每日剂量超过40 毫克/天(泼尼松当量)与发生股骨头坏死的风险较高有关。一项前瞻性研究表明,接受平均每日皮质类固醇激素剂量大于40 mg的患者的股骨头坏死发生率高出4 倍(P < 0.05)。Nagasawa等在一项对45名诊断为SLE并接受口服泼尼松龙(40 mg/天)治疗的患者进行的研究中,观察到5年内与股骨头坏死的剂量-反应关系。根据每年的MRI扫描,他们发现在早期股骨头坏死患者中使用高剂量皮质类固醇(> 1000 mg/天)的频率高于没有股骨头坏死的患者(87% 对 37%;P < 0.01)。研究之间的差异可能是由于最近增加了MRI用于诊断早期股骨头坏死,而这在放射学X线片评估中可能无法检测到。
多项研究还报告了累积皮质类固醇激素剂量与股骨头坏死之间的关联。例如,先前的报告表明,接受大于2 g累积皮质类固醇激素剂量的患者发生股骨头坏死的风险较高。然而,尚不清楚这是否代表患者以相同比率发生股骨头坏死的上限与总剂量或下限无关,在该下限以上,股骨头坏死的风险会随着累积剂量的增加而增加。此外,Nakamura 等对201名 SLE 患者进行了 13 年以上的评估,并观察到股骨头坏死风险与皮质类固醇激素累积剂量增加有关,15% 的患者由于增加皮质类固醇激素剂量而发展为股骨头坏死 [39]。Shibatani及其同事评估了经历多个排斥周期的肾移植受者,他们证明了股骨头坏死和累积皮质类固醇激素剂量之间的关联(OR 4.2;P = 0.008),但与排斥事件数无关,这暗示了更高累积剂量的可能性,而不是与宿主抗移植物疾病的全身效应相比,可能与股骨头坏死发病机制有关[60]。然而,难以准确区分平均每日皮质类固醇激素剂量和累积剂量对股骨头坏死风险的影响,因为具有最高累积剂量的患者通常接受更高的平均每日剂量和/或接受更长的治疗时间。
治疗持续时间也被认为是发生骨坏死的独立危险因素。Nakamura等评估了201名SLE患者(537 个关节),这些患者接受了每天超过 40 mg 的泼尼松治疗。在537个关节中,238 个(44%)发生了骨坏死。他们得出结论,骨坏死的进展与更高剂量的皮质类固醇治疗时间更长有关。
据报道,脉冲皮质类固醇激素治疗与股骨头坏死之间的关联是可变的。Oinuma 等对 72 名 SLE 患者进行了研究,发现在接受甲泼尼龙脉冲治疗和每天 40 毫克最低皮质类固醇激素剂量联合治疗的患者中,骨坏死发生率没有差异。在 32 名发生 ON 的患者中,17 名接受了脉冲皮质类固醇治疗,而在 40 名未发生骨坏死的患者中,18 名接受了脉冲皮质类固醇治疗。然而,这项研究没有具体说明脉冲皮质类固醇的剂量。然而,在 Ce 等的一项研究中,60 名没有任何骨坏死危险因素的多发性硬化症患者接受了脉冲皮质类固醇治疗,并与未接受皮质类固醇治疗的一组患者进行了比较。治疗组接受的累积脉冲糖皮质激素剂量大于10 g,他们只接受脉冲糖皮质激素,脉冲之间没有任何维持剂量。据观察,与非皮质类固醇治疗组(0%)相比,经MRI 诊断的治疗患者股骨头坏死的发生率(15.5%;P < 0.05)显着更高。在一项对 498 名肾移植患者的研究中观察到了类似的结果,该研究表明,与接受非脉冲治疗的患者(3%;P < 0.01)相比,接受脉冲治疗的患者(11%)的骨坏死发生率显着更高(3%;P < 0.01)。
这项研究有几个局限性。缺乏前瞻性随机对照试验可能导致研究设计和个别报告中的报告偏倚,这可能会扭曲观察到的结果。尽管双盲、前瞻性随机对照试验是循证医学的金标准,但在某些情况下,例如肾移植或 SLE,一线治疗是皮质类固醇,设计拒绝治疗的研究是不道德的。因此,我们主要依靠病例对照研究。骨坏死诊断方法也缺乏一致性。 1990 年之后发表的研究使用了 MRI,而早期的研究依赖于患者症状、放射学检查结果、活检和骨扫描,由于诊断敏感性低 [78] 可能低估了真正的骨坏死发生率。此外,由于统计分析的自由度不足,对肾移植或 SLE 以外的医学诊断的低质量研究阻碍了多变量分析。这在对骨髓移植受者、心脏移植和 SARS 的研究中很明显。然而,我们能够分析两个常见的患者组(SLE 和肾移植)。此外,几乎所有研究都报告使用高累积皮质类固醇剂量。该研究没有考虑偶然的皮质类固醇剂量,例如使用通常与骨坏死无关的最小剂量的剂量包或皮质类固醇。我们专注于接受最低累积剂量为 2 g 或 30 mg 每日剂量少于 2 个月的患者。因此,这些结果可能并不表明患者在与骨坏死没有直接关系的医疗条件下给予低累积剂量(< 2 g)的皮质类固醇。
这些对现有文献的荟萃分析和评估表明,大剂量皮质类固醇激素治疗可能会使发生股骨头坏死的风险增加10倍。接受每日剂量> 40 mg治疗的患者风险更高,剂量每增加10 mg,发病率增加3.6%。累积皮质类固醇激素剂量和治疗持续时间的影响不太清楚,但可能与每日剂量和潜在诊断具有协同关系。脉冲治疗对增加骨坏死风险有影响。随着这种疾病的分子和遗传基础的发展,通过基于MRI的前瞻性研究进一步了解骨坏死的危险因素将有助于医生对患者进行教育。目前,我们建议谨慎行事并使用尽可能低的皮质类固醇激素剂量,同时仍保持临床疗效并将风险降至最低。
High-Dose Corticosteroid Use and Risk of Hip Osteonecrosis: Meta-Analysis and Systematic Literature Review.
Abstract
The effect of varying corticosteroid regimens on hip osteonecrosis incidence remains unclear. We performed a meta-analysis and systematic literature review to determine osteonecrosis occurrences in patients taking corticosteroids at varying mean and cumulative doses and treatment durations, and whether medical diagnoses affected osteonecrosis incidence. Fifty-seven studies (23,561 patients) were reviewed. Regression analysis determined significance between corticosteroid usage and osteonecrosis incidence. Osteonecrosis incidence was 6.7% with corticosteroid treatment of >2 g (prednisone-equivalent). Systemic lupus erythematosus patients had positive correlations between dose and osteonecrosis incidence. Each 10 mg/d increase was associated with a 3.6% increase in osteonecrosis rate, and >20 mg/d resulted in a higher osteonecrosis incidence. Clinicians must be wary of osteonecrosis in patients on high corticosteroid regimens, particularly in systematic lupus erythematosus.
Keywords: corticosteroid; hip; meta-analysis; osteonecrosis; risk factors.
Osteonecrosis can lead to destructive arthropathies affecting the hip, knee, shoulder, and other joints, and it occurs most commonly in the first four decades of life 1., 2., 3.. This disease represents 2% to 10% of total hip arthroplasties performed in the United States and Europe, but may be as high as 50% to 60% in Korea and Japan 1., 4., 5., 6.. The etiology of atraumatic osteonecrosis remains multifactorial, and no consensus exists on common pathophysiologic mechanisms. Vascular impairment, abnormal cellular reparative processes, and genetic point mutations have been implicated 7., 8., 9., 10.. Risk factors include direct causes such as trauma, radiation exposures, hematologic diseases (sickle cell), and dysbarism (Caisson disease), as well as numerous indirect associated factors, such as rheumatologic or metabolic diseases, corticosteroids, alcohol, and/or smoking 1., 2., 3., 7..
Heimann and Freiberger [11] were among the earliest to report cases of osteonecrosis in patients treated with high corticosteroid doses. Multiple studies since then have implicated prolonged, high-dose corticosteroid use as an independent factor associated with osteonecrosis, and it has been reported that doses greater than 2 g within three-months present a risk for developing osteonecrosis 3., 12.. However, there are marked heterogeneities in patient demographics and epidemiologic variabilities between studies. Furthermore, few reports have examined differences in osteonecrosis incidences as functions across different medical diagnoses.
A systematic literature review and a meta-analysis were conducted to investigate the association of high-dose corticosteroid therapy with osteonecrosis incidences. Primary research questions were: (1) what were the overall osteonecrosis incidences in patients taking high-dose corticosteroids; (2) does the underlying disease for which corticosteroids are used affect osteonecrosis incidences; (3) whether mean doses, cumulative doses, or treatment durations were associated with incidences; and (4) whether pulsed therapies affected incidences.
Results
Incidence of Osteonecrosis
The systematic review demonstrated overall osteonecrosis incidence of 6.7% (range, 0.3% to 52%) in patients taking high-dose corticosteroids. Two level I studies proved a significant positive correlation between cumulative dose and the incidence of osteonecrosis, whereas, five level II studies failed to show it.
Disease and Incidence of Osteonecrosis
Osteonecrosis incidence for SARS was 21.8%, SLE 15.7%, renal transplant 14.7%, and BMT 6.6% (Fig. 2 ). Across all diagnoses, we observed positive associations between mean corticosteroid doses and osteonecrosis (Fig. 3 ). This was irrespective of underlying disease, as analysis of variance of osteonecrosis incidence between patients with different medical diagnoses (SLE, severe acute respiratory syndrome, bone marrow transplantation, renal transplantation) demonstrated no differences between diagnostic categories (P = 0.16). The regression analysis demonstrated a significant positive correlation in SLE patients (r = 0.81; R2 = 0.67; P < 0.05), however, this was not significant in renal transplant recipients (r = 0.32; R2 = 0.09; P > 0.05). It was also noted that renal transplant recipients and SLE patients were more likely to develop osteonecrosis if they were younger than 35 years compared to those who were older (22 versus 13%; P = 0.04, and 33 versus 7%; P = 0.02, respectively).
Meta-analysis of osteonecrosis in patients treated with greater than 20 mg per day demonstrated significantly higher odds than less than 20 mg per day corticosteroid users (OR 9.1; 95% confidence interval, 4.6 to 19.8) (Fig. 4A). For patients treated with high cumulative corticosteroid doses (greater than 10 g), the odds ratio for developing osteonecrosis was 2.4 (95% CI. 0.8 to 6.4), and lower dosing regimens were associated with a lower osteonecrosis incidence (OR 0.4; 95%, confidence interval 0.25 to 0.54) (Fig. 4B). Additionally, we observed that 10 mg per day dose increases resulted in a 3.6% increase in the rate of osteonecrosis. Due to the lack of data and control groups, the meta-analysis could not be performed comparing other dosing regimens or treatment duration effects on osteonecrosis risks.
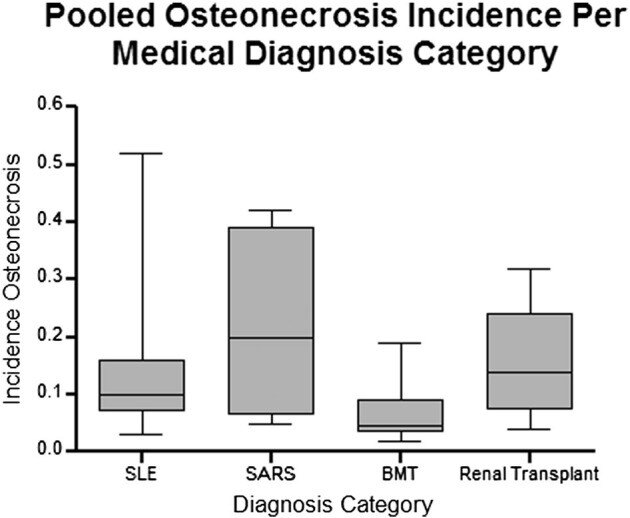
Fig. 2 Box-plot of pooled osteonecrosis incidence in patients treated with corticosteroids compared across multiple different primary medical diagnoses based on systematic review data. No significant difference is observed between groups (ANOVA; P = 0.158). SLE: systemic lupus erythematosus; SARS: severe acute respiratory syndrome; BMT: bone marrow transplant.
图2 基于系统评价数据的多个不同初级医学诊断的皮质类固醇(激素)治疗患者合并股骨头坏死发生率的箱线图。组间未观察到显着差异(ANOVA;P = 0.158)。SLE:系统性红斑狼疮; SARS:严重急性呼吸系统综合症;BMT:骨髓移植。
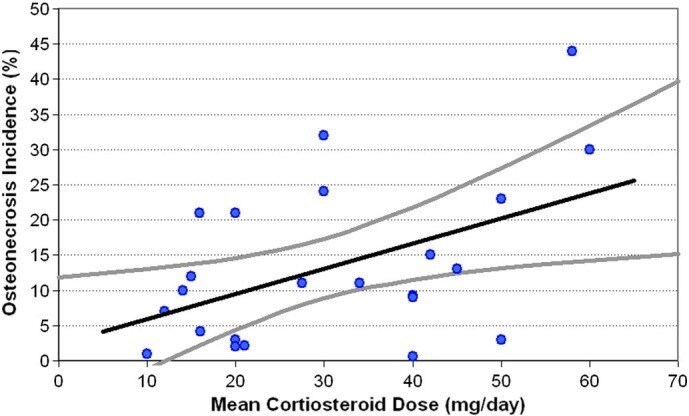
Fig. 3 Scatter plot of osteonecrosis incidence as a function of mean daily corticosteroid dose, irrespective of underlying medical disease or diagnosis based on systematic review data. Black line represents the linear regression model; gray lines represent the 95% confidence interval of the model.
Mean Dose, Cumulative Dose, Duration of Treatment and Osteonecrosis Incidence
图3 股骨头坏死发生率的散点图作为平均每日皮质类固醇(激素)剂量的函数,与基于系统评价数据的潜在内科疾病或诊断无关。黑线代表线性回归模型;灰线代表模型的95%置信区间。
平均剂量、累积剂量、治疗时间和股骨头坏死发生率
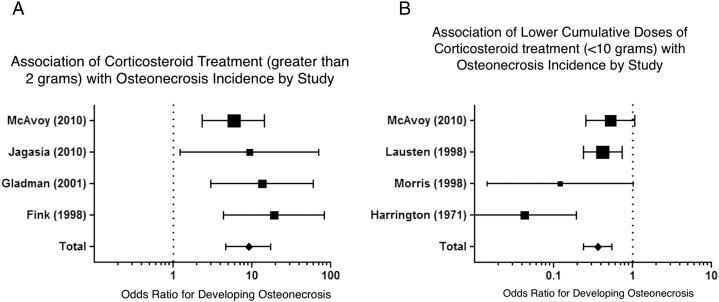
Fig. 4 (A and B) Comparison studies of osteonecrosis incidence included in the meta-analysis: (A) patients who did not receive any corticosteroids and those receiving greater than 2 g of corticosteroids; and (B) patients treated with less than 10 g of cumulative corticosteroid treatment (all patients received at least 2 g of corticosteroid). Horizontal error bars represent the 95% confidence interval of the odds ratio for each individual study. The diamond represents the pooled odds ratio for all the studies with a corresponding horizontal bar representing the 95% confidence interval. A pooled odds ratio > 1 represents higher-odds of having osteonecrosis if treated with greater than 2 g of corticosteroid (A) while an odds ratio of less than 1 is associated with lower odds of having osteonecrosis if treated with less than 10 g of corticosteroid (B).
图4(A和B)荟萃分析中股骨头坏死发生率的比较研究:(A)未接受任何皮质类固醇激素的患者和接受大于2g皮质类固醇激素的患者;(B)接受少于10 g累积皮质类固醇激素治疗的患者(所有患者接受至少2 g皮质类固醇激素)。水平误差条代表每个单独研究的优势比的95%置信区间。菱形代表所有研究的汇总优势比,相应的水平条代表95%置信区间。合并优势比 > 1 表示如果使用大于2 g的皮质类固醇激素(A)治疗发生股骨头坏死的几率较高,而如果使用少于10 g的皮质类固醇激素治疗,小于1的优势比与发生骨股骨头坏死的几率较低相关(B)。
Cumulative doses and treatment durations had negative associations with incidence for both SLE and renal transplant patients. In SLE patients, cumulative dose and the duration of treatment showed negative trends (r = ? 0.85, R2 = 0.65 and r = ? 0.53, R2 = 0.29, respectively) with the incidence of osteonecrosis, but these were not significant (P > 0.05) and may not represent a true trend. In renal transplant recipients, there was no evidence of a significant correlation between cumulative doses and incidence of osteonecrosis (r = 0.31; R2 = 0.42; P > 0.05).
Pulsed Corticosteroid Therapy and Incidence of Osteonecrosis
Mean osteonecrosis incidence was 33% in twenty studies evaluating the effects of pulsed corticosteroids based on data from the systematic review.
Discussion
Our aim was to evaluate the available literature and to assess the association between corticosteroids and hip osteonecrosis, utilizing statistical methodologies. In particular, we assessed the effect of dosing regimens and treatment durations, as well as the role of different disease entities. This study builds upon individual reports that have demonstrated independent risk factors for osteonecrosis, which were not observed in earlier studies 2., 20., 66., 72.. Our results showed that osteonecrosis incidences were affected by treatment with corticosteroids, corticosteroid doses, and patient age. Specifically, patients treated with high-dose corticosteroids may be up to ten times as likely to develop osteonecrosis, and cumulative doses greater than 10 g may increase the likelihood of developing osteonecrosis by two-fold, compared to cumulative doses less than 10 g. In addition, it was observed in the regression analysis that the correlation between corticosteroid dose and osteonecrosis incidence was most evident in SLE patients. However, no differences between diagnoses were noted using the analysis of variance, and further study is needed before a stronger conclusion can be drawn.
Multiple studies have demonstrated that corticosteroids are independent risk factors for osteonecrosis. Shibatani et al, in a study of 150 patients, noted a significant association between the total dose of corticosteroids and osteonecrosis incidence in patients during the first two months following renal transplantation (OR = 4, P = 0.02) [60]. Nakamura et al reported a 10.3 odds ratio of developing osteonecrosis in SLE patients, which compared similarly with the results of the present study (OR = 9.1) [73]. We also observed strong correlations (R2 > 0.8) between mean daily corticosteroid doses, cumulative doses, and treatment durations and osteonecrosis incidences. However, no single factor predicted variability in osteonecrosis incidences, which pointed to possible synergistic effects between all three factors.
The underlying diagnoses may potentially affect the risk for developing osteonecrosis. However, it is unclear which plays the dominant role, the underlying disease or the effects of corticosteroids, which may have stronger negative synergistic effects for some disorders compared to others. A prospective magnetic resonance imaging (MRI) study by Shigemura et al demonstrated that SLE patients had significantly higher risk (RR 2.1) of osteonecrosis than non-SLE patients (37 versus 21%; P = 0.001) [66]. However, they excluded organ transplant recipients due to higher mortality rates, and relied primarily on other systemic inflammatory diseases (e.g. inflammatory bowel diseases, vasculitides, dermatologic autoimmune diseases) as comparison groups. Furthermore, Leiberman et al reported low incidences (3%) of osteonecrosis diagnosed with MRI at a mean of 31 months post-liver transplantation [47]. Presently, the true effect of underlying disease on osteonecrosis incidence remains to be determined, and additional prospective studies are needed.
In addition to differences in diagnosis, it is important to highlight the effects of demographic factors and race on the development of osteonecrosis. Although it is difficult to specify osteonecrosis rates in a whole population, some studies have evaluated osteonecrosis in Asian populations and have shown higher disease incidence, particularly in those who have corticosteroid-dependent conditions. For example, Yamaguchi et al demonstrated a rising trend in the incidence of nontraumatic ON in Japanese patients, particularly in those who had corticosteroid-dependent conditions, with rates of up to 44% in SLE patients who were on high-dose corticosteroids [74]. However, it was inconclusive whether this was a true trend or just an improvement in diagnostic capabilities. In addition, Fukushima et al observed that according to the Research Committee on Idiopathic Osteonecrosis of the Femoral Head in Japan, roughly 2200 new patients per year were diagnosed with ON. Comparatively, United States-based studies have shown much lower ON rates, with roughly a 15% ON prevalence in SLE patients [75]. Race-specific differences in gene mutations, such as factor V Leiden and prothrombin, have been implicated, however, definitive causes for disparities in incidence remain unclear, and may also be idiopathic or attributed to lifestyle [76]. Nevertheless, it is still important to highlight that there may be fundamental differences in the way certain patients respond to corticosteroids.
Several studies published in the last two decades have demonstrated associations between high corticosteroid doses and osteonecrosis incidences, although others did not have this trend 12., 42., 50.. Similar to results observed in this meta-analysis, a prior systematic review found a 4.6% increase in the rate of osteonecrosis for every 10 mg per day increase in corticosteroid doses (Fig. 2; present study had 10% increase per 10 mg/day) [67]. In addition, mean daily doses of greater than 40 mg per day (prednisone-equivalent) have been associated with a higher risk for developing osteonecrosis. One prospective study demonstrated 4-fold higher incidences of osteonecrosis in patients treated with mean daily corticosteroid doses of greater than 40 mg (P < 0.05) [66]. Nagasawa et al, in a study of 45 patients diagnosed with SLE and treated with oral prednisolone (40 mg/day), observed dose–response relationships with osteonecrosis over five years [77]. Based on yearly MRI scans, they found that administration of high corticosteroid doses (> 1000 mg/day) was more frequently found in patients with early stage osteonecrosis than those without osteonecrosis (87% versus 37%; P < 0.01). It is possible that discrepancies between studies may be attributed to recent increases in MRI use for the diagnosis of early-stage osteonecrosis, which may have otherwise been undetectable on radiographic assessment.
Multiple studies have also reported on the association between cumulative corticosteroid doses and osteonecrosis. For example, previous reports suggest that patients receiving greater than 2 g of cumulative corticosteroid doses are at a higher risk for developing osteonecrosis 1., 3.. However, it is unclear whether this represents a ceiling above which patients develop osteonecrosis at the same rates irrespective of total doses, or a floor, above which risk for osteonecrosis increases with higher cumulative doses. Additionally, Nakamura et al evaluated 201 patients with SLE over 13 years and observed that osteonecrosis risk was associated with increasing cumulative corticosteroid doses, with 15% of patients requiring increased corticosteroid doses developing the disease [39]. Shibatani and colleagues evaluated renal transplant recipients who underwent multiple rejection cycles, and they demonstrated associations between osteonecrosis and cumulative corticosteroid doses (OR 4.2; P = 0.008), but not with rejection episode numbers, which allude to the likelihood that higher cumulative doses, rather than systemic effects of host-versus-graft disease, may be implicated in osteonecrosis pathogenesis [60]. However, accurate differentiation between effects of mean daily corticosteroid doses and cumulative doses on the osteonecrosis risk is difficult, since patients with the highest cumulative doses often receive higher mean daily doses and/or are treated for longer durations.
Treatment durations have also been implicated as independent risk factors for developing osteonecrosis. Nakamura et al evaluated 201 patients (537 joints) with SLE who were treated with prednisone doses of greater than 40 mg per day [39]. Of the 537 joints, 238 (44%) developed osteonecrosis. They concluded that progression of osteonecrosis was associated with higher doses of corticosteroid treatment for longer durations.
The association between pulsed corticosteroid therapy and osteonecrosis has been reported to be variable. Oinuma et al, studying 72 patients with SLE, found no differences in osteonecrosis incidences in patients treated with pulsed methylprednisolone therapy in combination with minimum daily corticosteroid doses of 40 mg/day [38]. Of the 32 patients who developed ON, 17 were treated with pulsed corticosteroids while of the 40 patients who did not develop osteonecrosis, 18 were treated with pulsed corticosteroids. However, this study did not specify pulsed corticosteroid dosages. However, in a study by Ce et al, 60 multiple sclerosis patients, who did not have any risk factors for osteonecrosis, were treated with pulsed corticosteroids and were compared to a matched group of patients who did not receive corticosteroids [72]. Cumulative pulsed corticosteroid doses received by the treatment group was greater than 10 g, and they only received pulsed-corticosteroids and were not treated with any maintenance doses between pulses. It was observed that treatment patients had significantly higher incidences of femoral head osteonecrosis as diagnosed on MRI (15.5%; P < 0.05) compared to the non-corticosteroid group (0%). Similar results were observed in a study of 498 renal transplant patients, which demonstrated a significantly greater incidence of osteonecrosis in those receiving pulsed therapies (11%) compared to patients receiving non-pulsed therapy (3%; P < 0.01) [55].
There were several limitations of this study. The lack of prospective randomized–controlled trials may have contributed to both study-design and reporting bias in individual reports, which may have skewed the observed outcomes. Although double-blinded, prospective randomized–controlled trials are gold standards for evidence-based medicine, in certain situations, such as renal transplantation or SLE, where the first-line therapy is corticosteroids, it would have been unethical to design studies denying treatment. Thus, we relied primarily on case–control studies. There was also a lack of consistency in osteonecrosis diagnostic methods. Studies published after 1990 utilized MRI, while earlier studies relied on patient symptoms, radiographic findings, biopsies, and bone scanning, which due to low diagnostic sensitivity [78] may have underestimated the true osteonecrosis incidence. In addition, lower quality studies on medical diagnoses other than renal transplantation or SLE prevented multivariate analysis due to insufficient degrees of freedom in the statistical analyses. This was evident in studies on bone marrow transplant recipients, cardiac transplants, and SARS. However, we were able to analyze two common patient groups (SLE and renal transplantation). Furthermore, almost all studies reported using high cumulative corticosteroid doses. This study did not consider incidental corticosteroid doses, such as the use of dose-packs or corticosteroids in minimal doses that are typically not associated with osteonecrosis. We have focused on patients who received minimum cumulative doses of 2 g or 30 mg daily doses for less than 2 months. Thus, these results may not be indicative of patients given low cumulative doses (< 2 g) of corticosteroids for medical conditions that were not directly associated with osteonecrosis.
These meta-analysis and assessment of the available literature demonstrated that high-dose corticosteroid treatments may increase risk for developing osteonecrosis up to ten-fold. Patients treated with daily doses > 40 mg were at higher risk, with 3.6% increase in incidences for every 10 mg increase in doses. Effects of cumulative corticosteroid doses and treatment durations are less clear, but are likely to have synergistic relationships with daily doses and underlying diagnoses. Pulsed-therapy has effects on increasing osteonecrosis risk. As molecular and genetic bases for this disease evolves, further knowledge of risk factors for osteonecrosis with prospective MRI-based studies will assist medical practitioners in educating patients. Presently, we would advise erring on the side of caution and using the lowest possible corticosteroid doses, while still maintaining clinical efficacy and minimizing risks.
文献出处:Michael A Mont, Robert Pivec, Samik Banerjee, Kimona Issa, Randa K Elmallah, Lynne C Jones. High-Dose Corticosteroid Use and Risk of Hip Osteonecrosis: Meta-Analysis and Systematic Literature Review. Review J Arthroplasty. 2015 Sep;30(9):1506-1512.e5. doi: 10.1016/j.arth.2015.03.036. Epub 2015 Apr 8.
本文是陶可版权所有,未经授权请勿转载。本文仅供健康科普使用,不能做为诊断、治疗的依据,请谨慎参阅





评论