 三甲
三甲
干细胞治疗股骨头坏死:最新进展及未来研究发展趋势
干细胞治疗股骨头坏死:最新进展及未来研究发展趋势
作者:Lei Zhao, Alan David Kaye, Aaron J Kaye, Alaa Abd-Elsayed.
作者单位: Department of Orthopedics, Shandong Provincial Hospital Affiliated with Shandong University, Jinan, 250021, China.
译者:陶可(北京大学人民医院骨关节科)
摘要
综述目的:股骨头坏死(ONFH)是一种常见病、多发病。它是由不同病因导致的血液供应中断引起的。股骨头坏死(ONFH)导致股骨头软骨下骨变性、坏死,最终导致股骨头塌陷。股骨头坏死(ONFH)致残率较高,严重影响患者生活质量,且常累及中青年人群。
最新发现:近年来,干细胞和再生医学的技术和理解不断发展。许多研究报告了干细胞移植治疗股骨头坏死(ONFH)的成功结果。因此,干细胞移植有望成为治疗股骨头坏死(ONFH)的新方法。因此,在本报告中,我们评估了利用干细胞治疗股骨头坏死(ONFH)的现有技术和结果。通过计算机在线检索2006年1月至2017年6月期间的PubMed和Cochrane图书馆数据库,使用英文关键词“治疗、干细胞、股骨头坏死”检索相关文章。选取与股骨头坏死(ONFH)治疗相关的文献。我们的检索共获得 161 篇文章,但只有 9 篇文章符合我们的纳入标准并被纳入我们的报告中。
本综述表明,细胞技术在治疗股骨头坏死(ONFH)方面已显示出良好的证据。然而,这项技术还需要进一步深入研究,以更好地探索和解决更理想的方法来克服与细胞来源相关的困难。
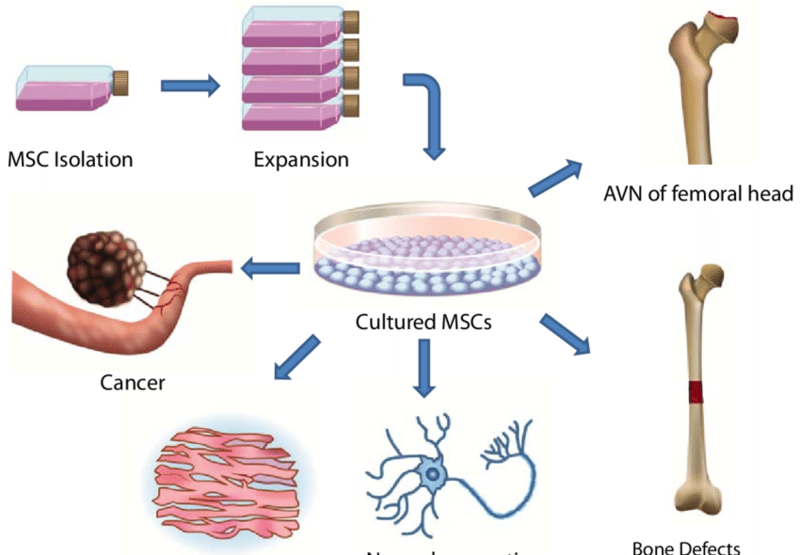
图示:干细胞技术被广泛用于治疗股骨头坏死(ONFH)、骨缺损、神经退行性病变等相关疾病
手术治疗
如果股骨头坏死(ONFH)进展迅速且非手术治疗无效,则需要手术治疗。外科手术主要包括两大类:股骨头本身重建和全髋关节置换术(THA)。保留股骨头的手术技术包括:髓芯减压、截骨术以及有或无血供的植骨[36]。
髓芯减压是治疗早期股骨头坏死(ONFH)的重要手术方法之一。主要是通过髓芯减压突破硬化区,导致骨内压降低、血液供应改善[37]。髓芯减压更适合早期股骨头坏死(ONFH)患者。一些临床医生发明了血管化和非血管化骨移植物,或设计了用其他材料(例如钽棒)支撑的支架治疗股骨头坏死(ONFH) [38]。
目前植骨治疗股骨头坏死(ONFH)的适应症有:(1)带血管腓骨移植;(2)带血管蒂髂骨移植术;(3)游离腓骨移植;(4)骨瓣移植;(5)病灶清除、打压植骨手术;(6)病灶切除、记忆合金网植入;(7)病灶切除、打压植骨、多孔钽钉支撑手术。
各种植骨都存在问题,因此多孔钽棒得到了广泛的应用。与传统的单一打压植骨相比,多孔钽棒支撑组合在I-II期股骨头坏死(ONFH)中具有明显优势。一项研究发现,股骨头髓芯减压联合植骨、2mm打压植骨加多孔钽棒支撑等手术方法对股骨头坏死(ONFH)患者,可延长股骨头寿命,推迟全髋关节置换术(THA)时间[39]。
截骨术的目的是去除股骨头承重区的坏死区。截骨术包括股骨转子旋转的内翻或外翻截骨术等,以不改变股骨髓腔为原则[40, 41]。
如果股骨头塌陷严重(ARCO分期、IIIC和IV),并且关节功能严重丧失或中度疼痛,应选择全髋关节置换术(THA)。
传统治疗结合干细胞治疗股骨头坏死(ONFH)
干细胞MSCs来源于中胚层间充质,广泛分布于骨髓、骨膜、肌肉、滑膜、滑液、肝脏、外周组织、脐带血、脂肪、胎盘、胎肺、胎肾、脐带等组织中[42, 43]。从这些组织中分离提取出具有多向分化能力的间充质干细胞,细胞具有很强的自我更新和增殖能力[44]。它们在特定的诱导条件下可以分化为骨、软骨、脂肪组织、肌肉、肌腱和血管内皮细胞,甚至可以分化为神经细胞[45]。相关研究表明干细胞MSC同种异体移植不会发生排斥反应[46]。它已广泛应用于干细胞移植、基因治疗、组织工程和其他研究工作。
对于许多难治性疾病,细胞移植似乎是一种相对有效的疗法。移植的自体细胞通常具有特定的功能,包括修复受损区域,避免使用传统药物对身体产生的毒性作用或副作用[47]。
间充质干细胞已广泛应用于骨缺损、软骨缺损、组织损伤、骨关节炎、股骨头坏死等修复。间充质干细胞也已应用于临床实践的其他领域[48]。干细胞治疗股骨头坏死(ONFH)是当前研究的热点,研究表明干细胞在特定条件下可以分化为成骨细胞。成骨细胞在骨坏死修复过程中发挥着重要作用,研究表明,使用骨髓干细胞移植到坏死股骨头中可以使股骨头坏死(ONFH)的发生率降低至0%[49]。
髓芯减压联合干细胞MSC移植
髓芯减压后的干细胞MSC移植可以为坏死股骨头的修复和重建提供种子细胞[16]。
Tabatabaee等的一项研究中[14],在髓芯减压的基础上,对 18 例 28 髋的股骨头坏死(ONFH)患者进行自体骨髓移植,所有患者平均随访 2 年。所有患者的西安大略大学和麦克马斯特大学平均骨关节炎指数(WOMAC)和视觉模拟量表(VAS)评分均显着改善。磁共振成像(MRI)显示联合治疗组有显着改善。研究人员认为,这项技术可用于改善股骨头坏死区域的修复,至少在股骨头坏死(ONFH)的早期阶段是这样。Rastogi等[13]也得出结论,髓芯减压联合自体骨髓干细胞植入是一种安全有效的方法。Sen等进行了一项临床对照研究,表明在髓芯减压后将自体骨髓单核细胞注入核心道可以为治疗股骨头坏死(ONFH)带来更好的临床结果和髋关节存活率[15]。Ma等[18]评价基于髓芯减压的骨髓血沉棕黄层(BBC)植入治疗股骨头坏死的效果。 作者得出结论,在治疗的第二年,92%的股骨头坏死(ONFH)患者的髋关节功能得到改善。更重要的是,治疗组ARCO分期 I/II期髋部无进展率为100%,对照组为66.7%[18]。Zhang等等[17]指出,髓芯减压联合自体骨髓间充质干细胞移植可以减轻疼痛,延缓或避免股骨头塌陷。MRI评估发现软骨病变面积显着缩小,软骨质量显着改善。Gangji等[19]比较了核心减压联合含有间充质干细胞(MSCs)或自体成骨细胞(OB)的骨髓浓缩物(BMC)治疗股骨头坏死(ONFH)的效果。他们发现,除了减轻股骨头坏死(ONFH)的疼痛之外,自体成骨细胞(OB)植入在延缓骨循环协会(ARCO) III期进展方面比干细胞BMC治疗更有效。然而,其他研究人员却得出了不同的结论。Lim等[11]进行了一项对照试验,比较了两组治疗股骨头坏死的临床效果和影像学结果:一组采用多次钻孔减压联合干细胞移植,另一组采用干细胞移植联合其他髓芯减压方法:刮除术、植骨术等。结论是两组之间没有统计学上的显着差异。从本研究来看,对于ARCO I-II期塌陷期的股骨头坏死(ONFH),髓核减压联合干细胞MSC移植获得了良好的手术效果,但对于塌陷后的ARCO III-IV期患者,这种手术方式效果不佳。
生物力学支持和干细胞MSC动脉输注
股骨头坏死(ONFH)发生后,尽管进行了髋关节挽救治疗,改善股骨头内的血液供应始终是该疾病的主要治疗方法之一。自体干细胞移植和血管再生技术可以在治疗中发挥重要作用。Kocher等[50]证实干细胞MSCs和内皮祖细胞可以促进缺血血流恢复。Kinnaird等[51]发现,将骨源干细胞肌肉注射到大鼠缺血后肢后,骨源干细胞可以促进侧支循环和肢体功能恢复。此外,与血管生成相关的细胞因子基因表达水平增加。Mao等[12]进行了一项对照试验,其中55例89髋的股骨头坏死(ONFH)患者接受钽棒植入联合间充质干细胞靶向动脉内灌注,所有患者均随访36个月。随访36个月时,联合治疗组中有3个髋关节(6.25%)被发现临床失败,需要进行全髋关节置换术THA;相比之下,对照组中有 9 个髋关节(21.95%)接受了全髋关节置换术THA。与对照组相比,联合治疗在36个月时显着改善了Harris 髋关节评分 (HHS)。他们的结论是,干细胞MSC动脉输注是治疗早期股骨头坏死(ONFH)的有效且安全的方法。与开放式股骨头干细胞移植相比,干细胞MSC选择性动脉输注具有干细胞存活更好、分化更好、操作创伤小、不良反应少、患者依从性好等优点。手术可达到以下效果:(1)疏通病变股骨头,改善静脉回流,降低骨内压,恢复或改善股骨头的血液供应。(2)改善或增加股骨头坏死区及髋关节周围组织的血液循环,为坏死股骨头提供良好的局部血液供应。(3) 干细胞MSCs可以保护局部血管内皮,促进血管内皮细胞的修复、再生和血管生成。随着干细胞干预的进一步研究,这种方法似乎对未来的股骨头坏死(ONFH)患者有积极作用。
骨组织工程和间充质干细胞
组织工程是应用细胞生物学,在体外培养功能相关的活细胞,复合移植体内组织缺损并完成修复和重建。干细胞MSCs具有较强的分化潜能和增殖能力,细胞在来源、分离方式、分化组织类型等方面对于修复股骨头坏死(ONFH)骨缺损具有独特的优势。因此,它是骨组织工程种子细胞最理想的选择。干细胞MSCs制成的复合支架可用于加强股骨头坏死区域的修复,但也可以用作细胞、基因和生长因子的生物载体。此外,间充质干细胞可以整合细胞和受体来调节细胞功能。
Kang等[52]报道采用自体髂骨松质骨移植联合间充质干细胞移植治疗股骨头坏死(ONFH)患者,随访32个月,临床疗效良好。Kawate等[53]采用β-磷酸三钙作为载体承载自体骨髓干细胞BMSCs植入股骨头坏死部位联合血管化腓骨移植治疗股骨头坏死(ONFH),长期随访发现大部分股骨头坏死部位患者缓解至影像学观察不同程度的局部高密度新骨形成。该疾病已得到控制,表明组织工程方法具有更好的治疗股骨头坏死(ONFH)的潜力。Yamasaki等[54]报道22例(30髋)股骨头坏死(ONFH)患者接受单核细胞联合磷酸钙治疗,8例(9髋)股骨头坏死(ONFH)患者仅接受磷酸钙治疗。平均随访时间为 29 个月。治疗组股骨头坏死面积减少,仅3髋(3/30)出现股骨头塌陷,而对照组6髋(6/8)出现严重股骨头塌陷。
Xiao等[55]在复合骨上用自体骨髓间充质干细胞结合重组人骨形态发生蛋白2修复了兔股骨头坏死(ONFH)缺损,他们发现这种方法可以防止塌陷并能形成新的骨组织。骨组织工程的兴起为干细胞MSCs治疗股骨头坏死(ONFH)提供了有力支持。
干细胞MSC移植联合细胞因子
随着更多研究确定股骨头坏死(ONFH)病因和发病机制,细胞因子已成为股骨头坏死(ONFH)治疗的焦点。目前已发现多种细胞因子可促进干细胞MSCs分化为骨细胞和软骨细胞[56]。股骨头坏死(ONFH)基础实验中使用的细胞因子包括骨形态发生蛋白(BMP)、血管内皮生长因子(VEGF)、碱性成纤维细胞生长因子(BFGF)、肿瘤坏死因子(TNF)等。这些因子可以诱导干细胞MSC不可逆地分化为成骨细胞,有的可以诱导新生血管形成并参与股骨头坏死(ONFH)的修复[57]。Reddi等[58]得出结论,骨形态发生蛋白(BMP)可以使间充质细胞首先分化为软骨细胞,然后分化为成骨细胞。成骨细胞可用于治疗股骨头坏死,这与它们在促进新骨形成、血运重建和促进关节软骨生长方面的作用有关。碱性成纤维细胞生长因子(BFGF)具有较强的成骨和诱导血管生成作用,与骨形态发生蛋白(BMP)联合应用效果优于单独应用骨形态发生蛋白(BMP)。
Jin等[59]通过碱性成纤维细胞生长因子(BFGF)和骨形态发生蛋白(BMP)作用于兔股骨头坏死(ONFH),观察到坏死区域新生骨小梁的形成,由此,作者得出结论,碱性成纤维细胞生长因子(BFGF)和骨形态发生蛋白(BMP)可以显着促进骨细胞和成纤维细胞的生长和增殖。血管生成因子促进股骨头坏死区域相对于正常区域的血运重建。血管生成因子可以维持坏死区域的血液供应,可能作为治疗股骨头坏死(ONFH)的有效手段。
血管内皮生长因子(VEGF)具有很强的促进内皮生长和血管生成的作用,其他血管生成因子通过增强血管内皮生长因子(VEGF)的表达和产生来全部或部分促进血管生成[60]。尽管许多研究证明生长和分化因子在骨坏死的治疗中发挥着重要作用,但这些研究都是在体外或动物实验中进行的,并没有直接证据证明它们在人体中的有效用途。在这方面,较新的治疗方法通常基于对病理过程的更好理解。随着对股骨头坏死(ONFH)过程了解的加深,各种生长和分化因子的作用将逐渐变得清晰,这种类型的治疗可能会在未来出现[61]。
Surgical Treatment
Surgical treatment is indicated if ONFH progresses rapidly and non-surgical treatment is not effective. The surgical procedures include two main categories: the reconstruction of the femoral head itself and THA. The operation to preserve the femoral head includes core decompression, osteotomy, and bone grafting with or without blood supply [36]. Core decompression is one of the important surgical methods for the treatment of early ONFH. It is mainly through core decompression to break through the hardening zone, leading to a reduction in intraosseous pressure and improvement of blood supply [37]. Core decompression is more suitable for early-stage patients with ONFH. Some clinicians have invented vascularized and non-vascularized bone grafts, or designed a support with other materials, such as tantalum rods [38]. At present, the indications for bone grafting are (1) vascularized fibular grafting; (2) with vascular pedicled iliac bone graft; (3) free fibula grafting; (4) with bone flap transplantation; (5) lesion removal, compression of bone graft surgery; (6) lesion removal, memory alloy mesh implantation; and (7) lesion removal, impaction bone graft, and porous tantalum nail support surgery. All kinds of bone grafting have issues, and thus, porous tantalum rod is widely used. Compared with traditional single compression osteogenesis, the combination of porous tantalum rod support has obvious advantages in stage I–II ONFH. A study found that the core decompression and bone implant, 2 mm impaction bone grafting plus porous tantalum rod supporting methods of operation in ONFH patients, can prolong the life of the femoral head and postpone THA time [39]. The purpose of osteotomy is to remove the necrotic zone from the weight-bearing area of the femoral head. Osteotomy includes varus or valgus osteotomy by the femoral rotor rotation osteotomy, and so on, with the principle of not changing the medullary cavity of the femur [40, 41]. If the femoral head collapse is serious (ARCO, IIIC and IV), and there is a severe loss of joint function or more moderate pain, THA should be chosen.
Traditional Treatment Combined with Stem Cell Therapy for ONFH
MSCs are derived from mesodermal mesenchyme and are widely distributed in the bone marrow, bone membrane, muscle, synovium, synovial fluid, liver, peripheral tissue, umbilical cord blood, fat, placenta, fetal lung, fetal kidney, umbilical cord, and other tissues [42, 43]. MSCs with multi-directional differentiation ability have been isolated and extracted from these tissues, and the cells have strong self-renewal and proliferation ability [44]. They can differentiate into bone, cartilage, adipose tissue, muscle, tendon, and vascular endothelial cells under specific conditions of induction and can even differentiate into nerve cells [45]. Related studies have revealed that MSC allogeneic transplantation does not undergo rejection [46]. It has been widely used in stem cell transplantation, gene therapy, tissue engineering, and other research endeavors.
For many refractory diseases, cell transplantation appears to be a relatively effective therapy. Transplanted autologous cells usually have specific functions, including repairing damaged regions, avoiding toxic effects on the body or side effects caused by the use of traditional drugs [47].
MSCs have been widely used in the repair of bone defects, cartilage defects, tissue injuries, osteoarthritis, and osteonecrosis of the femoral head. MSCs have also been applied in other fields of clinical practice [48]. Stem cells in the treatment of ONFH are the focus of current research, which has demonstrated that stem cells can differentiate into osteoblasts under specific conditions. Osteoblasts play an important role in the process of repairing bone necrosis, and studies have shown that using bone marrow stem cells transplantation into a necrotic femoral head leads to a decrease in the incidence rate of ONFH to 0% [49].
Core Decompression Combined with MSC Transplantation
MSC transplantation after core decompression can provide seed cells for the repair and reconstruction of a necrotic femoral head [16].
In a study by Tabatabaee et al. [14], based on core decompression, autologous bone marrow transplantation was performed on ONFH in 18 patients with 28 hips, and all patients were followed up for an average of 2 years. The mean Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and visual analog scale (VAS) scores in all patients improved significantly. Magnetic resonance imaging (MRI) showed a significant improvement in the combination treatment group. The researchers believe that this technique can be used to improve the repair of femoral head necrotic area, at least in the early stages of ONFH. Rastogi et al. [13] also concluded that core decompression combined with autologous bone marrow stem cell implantation is a safe and effective method. Sen et al. conducted a clinical controlled study, which showed autologous bone marrow mononuclear cell instillation into the core tract after core decompression can result in better clinical outcomes and hip survival for treating ONFH [15]. Ma et al. [18] evaluated the effect of bone marrow buffy coat (BBC) implantation based on core decompression for treatment of osteonecrosis of the femoral head. Authors concluded that during the second year of treatment, improvement in hip function was noticed in 92% of patients with ONFH. More importantly, the non-progression rate for stage I/II hips was 100% in the treatment group and 66.7% in the control group [18]. Chang et al. [17] indicated that the core decompression combined with autologous bone marrow MSCs transplantation can reduce pain and delay or avoid femoral head collapse. Assessment by MRI found that the cartilage lesion area was significantly reduced and the cartilage quality was improved significantly. Gangji et al. [19] compared core decompression combined with bone marrow concentrate (BMC) containing mesenchymal stem cells (MSCs) or autologous osteoblastic cells (OB) to treat ONFH. They found that OB cell implantation was more efficacious than BMC treatment in delaying the progression to Association Research Circulation Osseous (ARCO) stage III in addition to reducing pain in ONFH.
However, other researchers have had different conclusions. Lim et al. [11] conducted a controlled trial in which the clinical effects and radiological results of two groups of treatment for osteonecrosis of the femoral head were compared: One group utilized the use of multiple drilling core decompression combined with stem cell transplantation, and the other group utilized the application of other core decompression method, curettage, and bone grafting. The conclusion was that there was no statistically significant difference between the two groups. From this study, for the collapse of the ARCO stage I–II period of ONFH, core decompression and MSC transplantation obtained a good surgical outcome, but for post-collapse of the ARCO stage III–IV patients, this surgical approach was ineffective.
Biomechanical Support and MSCs Arterial Infusion
After ONFH and despite hip salvage treatment, improving blood supply within the femoral head is always one of the main treatments of the disease. Autologous stem cell transplantation and vascular regeneration technologies can play an important role in management. Kocher et al. [50] confirmed that MSCs and endothelial progenitor cells can promote ischemic blood flow recovery. Kinnaird et al. [51] found that after intramuscular injection of bone-derived stem cells into the ischemic hind limbs of rat, bone-derived stem cells could promote collateral circulation and limb function recovery. In addition, the level of cytokine gene expression associated with angiogenesis was increased. Mao et al. [12] conducted a controlled trial in which 55 patients with 89 hips of ONFH underwent tantalum rod implantation in combination with targeted intra-arterial infusion of MSCs, and all patients were followed up for 36 months. At 36 months of follow-up, three hips in the combined treatment group (6.25%) were found to be clinically failed and required THA; in contrast, nine hips (21.95%) received THA in the control group. Compared to the control group, combination treatment significantly improved the Harris hip score (HHS) at 36 months. They concluded that MSC arterial infusion is an effective and safe method for early ONFH. Compared with open femoral head stem cell transplantation, MSC selective arterial infusion has the advantages of better stem cell survival, better differentiation, less invasive operation, less adverse reaction, and good patient compliance. The operation may achieve the following effects: (1) Dredge the diseased femoral head, improve the venous return, decrease the intraosseous pressure, and restore or improve the blood supply of the femoral head. (2) Improve or increase the blood circulation of the tissues around the femoral head necrotic region and the hip, providing a good local blood supply for the necrotic femoral head. (3) MSCs can protect the local vascular endothelium and promote the repair, regeneration, and angiogenesis of vascular endothelial cells. With additional research using stem cell intervention, it appears this method will be positive for future ONFH patients.
Bone Tissue Engineering and MSCs
Tissue engineering is the application of cell biology, functionally related living cells, which cultured in vitro, composite transplantation in vivo tissue defect and complete restoration and reconstruction. MSCs have strong differentiation potential and proliferation ability, and the cells have unique advantages for repairing ONFH bone defects in terms of source, separation mode, and differentiated tissue type. Thus, it is the most ideal choice for bone tissue engineering seed cells. The composite scaffolds made of MSCs can be used to reinforce the repair of necrotic defects in the structure but can also be used as a biological carrier for cells, genes, and growth factors. In addition, MSCs can integrate cells and receptors to regulate cellular function.
Kang et al. [52] reported using autoiliac cancellous bone graft combined with MSC transplantation for ONFH patients with follow-up for 32 months demonstrating good clinical efficacy. Kawate et al. [53] used beta-tricalcium phosphate as the carrier load bearing autologous BMSCs implanted into the area of the head necrosis combined with vascularized fibular grafting for treatment of ONFH, and long-term follow-up found that most patients with osteonecrotic sites were relieved to varying degrees, imaging observed local high density of new bone formation. The disease was under control and indicated that the tissue engineering approach had a better potential for the treatment of ONFH. Yamasaki et al. [54] reported that 22 cases (30 hips) of ONFH patients were treated with mononuclear cells combined with calcium phosphate and eight cases (nine hips) of ONFH patients were treated with calcium phosphate only. The average follow-up was 29 months. In the treatment group, the femoral head necrotic area was reduced, and only three hips (3/30) developed femoral head collapse, while six hips (6/8) in the control group developed serious femoral head collapse.
Xiao et al. [55] repaired rabbit ONFH defects with autologous bone marrow MSCs on composite bone combined with recombinant human bone morphogenetic protein 2. They found that this method prevented the collapse and can form new bone tissue. The rise of bone tissue engineering provides a strong support for the treatment of ONFH by MSCs.
MSC Transplantation Combined Cytokine
With additional research identifying ONFH etiology and pathogenesis, cytokines have become a focus of ONFH therapy. At present, many cytokines have been found to promote the differentiation of MSCs into bone cells and chondrocytes [56]. The cytokines used in basic experiments in ONFH include bone morphogenetic proteins (BMP), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (BFGF), tumor necrosis factor (TNF), and others. These factors can induce MSCs irreversibly to differentiate into osteoblasts, and some can induce neovascularization and participate in repair of ONFH [57]. Reddi et al. [58] concluded that BMP can cause mesenchymal cells to first differentiate into chondrocytes and then differentiate into osteoblasts. Osteoblasts may be used in the treatment of femoral head necrosis related to their role in promoting new bone formation, revascularization, and promoting articular cartilage growth. BFGF has a strong role in osteogenesis and induced angiogenesis, combined with BMP effect is better than the application of BMP alone.
Jin et al. [59] through the BFGF and BMP role in rabbit ONFH, observed in necrotic regions new bone trabeculae and thus, authors concluded that BFGF combined with BMP can significantly promote growth and proliferation of bone cells and fibroblasts. Angiogenic factors promote revascularization of femoral head necrotic area relative to the normal regions. Angiogenic factors can maintain blood supply to necrotic regions and may be used as an effective means of treatment for ONFH.
VEGF has a strong role in promoting endothelial growth and angiogenesis, and other angiogenic factors promoting angiogenesis in whole or in part by enhancing the expression and production of VEGF [60]. Although many studies have demonstrated that growth and differentiation factors play a significant role in the treatment of bone necrosis, these studies were in vitro or in animal experiments, and there is no direct evidence to prove their effective use in humans. In this regard, newer methods of treatment are typically based on improved understanding of pathology processes. With improved understanding of the ONFH process, the effects of various growth and differentiation factors will gradually become clearer and this type of treatment may be seen in the future [61].
Stem Cell Therapy for Osteonecrosis of the Femoral Head: Current Trends and Comprehensive Review
Abstract
Purpose of review: Osteonecrosis of the femoral head (ONFH) is a common and frequently occurring disease. It is caused by interruption of blood supply with different etiologies. ONFH leads to degeneration and necrosis of the subchondral bone of the femoral head and eventually collapse of the femoral head. ONFH has a high disability rate, seriously affecting the quality of living of patients, and often involves middle-aged and younger people.
Recent findings: In recent years, the technology and understanding of stem cells and regenerative medicine have been developing rapidly. Numerous studies have reported successful results in the treatment of ONFH by stem cell transplantation. Thus, stem cell transplantation is expected to serve as a new method in the treatment of ONFH. In the present report, therefore, we evaluated current techniques and outcomes utilizing stem cells in the treatment of ONFH. A computer-based online search of PubMed and Cochrane Library databases between January 2006 and June 2017 was performed to search related articles using the keywords of “treatment, stem cell, osteonecrosis of the femoral head“ in English language. Literature related to the treatment of ONFH was selected. Our search obtained a total of 161 articles, but only 9 articles met our inclusion criteria and were included in our report. The present review reveals that cell technology has demonstrated good evidence in the treatment of ONFH. However, this technology needs additional in-depth study to better explore and appreciate more ideal ways to overcome difficulties associated with source of cells.
文献出处:Lei Zhao, Alan David Kaye, Aaron J Kaye, Alaa Abd-Elsayed. Stem Cell Therapy for Osteonecrosis of the Femoral Head: Current Trends and Comprehensive Review. Review, Curr Pain Headache Rep. 2018 May 3;22(6):41. doi: 10.1007/s11916-018-0700-x.
本文是陶可版权所有,未经授权请勿转载。本文仅供健康科普使用,不能做为诊断、治疗的依据,请谨慎参阅





评论