
糖皮质激素患者围手术期的评价与处理(2022)
糖皮质激素患者围手术期的评价与处理(2022)Perioperative Evaluation and Management of Patients on Glucocorticoids
Chen C S, Santhanam P, Morris-Wiseman L, Salvatori R, Hamrahian A H. Perioperative Evaluation and Management of Patients on Glucocorticoids[J]. J Endocr Soc, 2022,7(2): bvac185.
转载文章的原链接1:
https://pubmed.ncbi.nlm.nih.gov/36545644/
转载文章的原链接2:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9760550/
Abstract
Myriad questions regarding perioperative management of patients on glucocorticoids (GCs) continue to be debated including which patients are at risk for adrenal insufficiency (AI), what is the correct dose and duration of supplemental GCs, or are they necessary for everyone? These questions remain partly unanswered due to the heterogeneity and low quality of data, studies with small sample sizes, and the limited number of randomized trials. To date, we know that although all routes of GC administration can result in hypothalamic-pituitary-adrenal (HPA) axis suppression, perioperative adrenal crisis is rare. Correlation between biochemical testing for AI and clinical events is lacking. Some of the current perioperative management recommendations based on daily GC dose and duration of therapy may be difficult to follow in clinical practice. The prospective and retrospective studies consistently report that continuing the daily dose of GCs perioperatively is not associated with a higher risk for adrenal crises in patients with GC-induced AI. Considering that oral GC intake may be unreliable in the early postoperative period, providing the daily GC plus a short course of IV hydrocortisone 25 to 100 mg per day based on the degree of surgical stress seems reasonable. In patients who have stopped GC therapy before surgery, careful assessment of the HPA axis is necessary to avoid an adrenal crisis. In conclusion, our literature review indicates that lower doses and shorter duration of supplemental GCs perioperatively are sufficient to maintain homeostasis. We emphasize the need for well-designed randomized studies on this frequently encountered clinical scenario.
关于糖皮质激素(GCs)患者围手术期管理的无数问题仍在争论中,包括哪些患者有肾上腺功能不全(AI)的风险,补充GCs的正确剂量和持续时间是多少,或者是否对每个人都有必要?由于数据的异质性和低质量,研究样本量小,随机试验数量有限,这些问题在一定程度上仍未得到解答。到目前为止,我们知道虽然所有的GC给药途径都能导致下丘脑-垂体-肾上腺(HPA)轴抑制,但围手术期肾上腺危机是罕见的。AI的生化检测与临床事件之间缺乏相关性。目前一些基于每日GC剂量和治疗时间的围手术期管理建议在临床实践中可能难以遵循。前瞻性和回顾性研究一致报道,围手术期继续每日剂量的GC与GC诱导的AI患者肾上腺危象的高风险无关。考虑到术后早期口服GC摄入量可能不可靠,根据手术应激程度提供每日GC加25 ~ 100mg /天短疗程的静脉注射氢化可的松似乎是合理的。在手术前停止GC治疗的患者中,仔细评估下丘脑轴是必要的,以避免肾上腺危机。总之,我们的文献综述表明,低剂量和短时间的围手术期补充GCs足以维持体内平衡。我们强调需要对这种经常遇到的临床情况进行精心设计的随机研究。
Keywords:
perioperative evaluation, perioperative management, glucocorticoids, hypothalamic-pituitary-adrenal axis suppression, glucocorticoid-induced adrenal insufficiency, adrenal crisis
Glucocorticoids (GCs) are one of the most commonly prescribed drugs with an estimated use prevalence of approximately 1% of the US population [1]. They are very effective anti-inflammatory medications and considered first-line treatment for many autoimmune conditions. One important consequence of supraphysiological and/or long-term GC treatment is the potential for hypothalamic-pituitary-adrenal (HPA) axis suppression leading to GC-induced adrenal insufficiency (AI), which is associated with increased morbidity and mortality [2]. When associated with a stressor such as a surgical procedure, HPA axis suppression can result in adrenal crisis. This outcome was recognized in early-20th-century studies when adrenalectomized dogs experienced circulatory shock after laparotomy that could be prevented by administering GCs [3, 4]. In the 1950s, multiple reports described patients on chronic GC therapy for rheumatoid arthritis who died shortly after orthopedic surgery. Postmortem examinations consistently revealed bilateral adrenal atrophy, leading to the conclusion that the adrenal glands’ inability to respond to surgical stress was the cause of death [5–7]. The resultant concern about postoperative adrenal crisis in patients on GCs led to the routine use of high-dose perioperative GC replacement in clinical practice.
糖皮质激素(GCs)是最常用的处方药之一,估计约占美国人口的1%[1]。它们是非常有效的抗炎药物,被认为是许多自身免疫性疾病的一线治疗方法。生理上和/或长期GC治疗的一个重要后果是下丘脑-垂体-肾上腺(HPA)轴抑制的可能性,导致GC诱导的肾上腺功能不全(AI),这与发病率和死亡率增加有关[2]。当与外科手术等应激源相关时,下丘脑轴抑制可导致肾上腺危机。这一结果在20世纪早期的研究中得到确认,当时切除肾上腺的狗在剖腹手术后出现循环休克,可以通过给予GC来预防[3,4]。在20世纪50年代,有多篇报道描述了接受慢性GC治疗类风湿关节炎的患者在骨科手术后不久死亡。尸检一致显示双侧肾上腺萎缩,从而得出结论,肾上腺无法应对手术应激是死亡原因[5-7]。因此,对GCs患者术后肾上腺危机的担忧导致临床常规使用高剂量的围手术期GC替代。
Currently, there is little high-quality evidence supporting routine perioperative use of high-dose GCs [8–11]. While underdosing perioperative GCs may place patients at risk for cardiovascular collapse, high doses carry a risk of hyperglycemia, hypertension, opportunistic infections, bone loss in a state of immobility, venous thromboembolism, and poor wound healing [12–14]. This review outlines the key physiologic aspects of the stress response to surgery, the effect of different forms of GCs on the HPA axis, the evidence for perioperative GC administration, and our personalized approach to perioperative management in adults with GC-induced AI.
目前,支持围手术期常规使用大剂量GCs的高质量证据很少[8-11]。围手术期剂量不足的GCs可能使患者有心血管衰竭的风险,而大剂量的GCs则有高血糖、高血压、机会性感染、不活动状态下的骨质流失、静脉血栓栓塞和伤口愈合不良的风险[12-14]。这篇综述概述了手术应激反应的关键生理方面,不同形式的GC对HPA轴的影响,围手术期GC给药的证据,以及我们对成人GC诱导AI的围手术期治疗的个性化方法。
Regulation of the HPA Axis Perioperatively
The HPA axis is regulated by the classic negative feedback of cortisol as well as other neurohumoral inputs including vasopressin, the autonomic nervous system, inflammation, and opioids (Fig. 1). This results in an estimated production of about 5.7 mg/m2 or 9.9 mg of cortisol per day in healthy adults which could increase to >100 mg during times of major stress [8, 15–17]. In plasma, the majority of cortisol (approximately 90%) binds tightly to corticosteroid-binding globulin (CBG) and loosely to albumin (∼5%); the remaining (∼5%) is the active, free fraction [18, 19]. Free cortisol binds to the glucocorticoid receptors in the target cell‘s cytoplasm. After glucocorticoids bind to its receptor, this complex translocates to the nucleus regulating the transcription, inducing or repressing a multitude of genes [20]. Glucocorticoid receptors are ubiquitously distributed in the body, including the hypothalamus and pituitary, where cortisol exerts a negative feedback [21]. Other rapid, nongenomic effects have been recognized over the past decades. Known mechanisms thought to mediate these actions include GC interaction with nonspecific cellular membrane receptors, cytosolic GC receptor mediating nongenomic effects, and membrane-bound GC receptors [22]. These nongenomic effects can involve activation of distinct signaling pathways that includes cAMP, with activation of multiple kinases, increase in intracellular calcium concentrations; as well as intracellular receptors that targets mitochondrial gene expression, all of these with a recognized tissue specificity [23]. These nongenomic mechanisms of action are highly relevant in the development of new drugs with higher selectivity and better side-effect profile compared to the classic GCs targeting the genomic actions.
下丘脑轴受经典的皮质醇负反馈以及其他神经体液输入(包括抗利尿激素、自主神经系统、炎症和阿片类药物)的调节(图1)。这导致健康成人每天产生约5.7 mg/m2或9.9 mg皮质醇,在主要压力时期可能增加到>100 mg[8,15 - 17]。在血浆中,大部分皮质醇(约90%)与皮质类固醇结合球蛋白(CBG)紧密结合,与白蛋白松散结合(约5%);剩下的(约5%)是活性的游离部分[18,19]。游离皮质醇与靶细胞细胞质中的糖皮质激素受体结合。糖皮质激素与其受体结合后,该复合体易位到细胞核中调节转录,诱导或抑制多种基因[20]。糖皮质激素受体在机体中广泛分布,包括下丘脑和垂体,皮质醇在其中发挥负反馈作用[21]。在过去的几十年里,其他快速的非基因组效应也被认识到。已知介导这些作用的机制包括GC与非特异性细胞膜受体的相互作用,胞质GC受体介导的非基因组效应,以及膜结合GC受体[22]。这些非基因组效应可能涉及不同信号通路的激活,包括cAMP,多种激酶的激活,细胞内钙浓度的增加;以及针对线粒体基因表达的细胞内受体,所有这些都具有公认的组织特异性[23]。与靶向基因组作用的经典GCs相比,这些非基因组作用机制与开发具有更高选择性和更小副作用的新药密切相关。
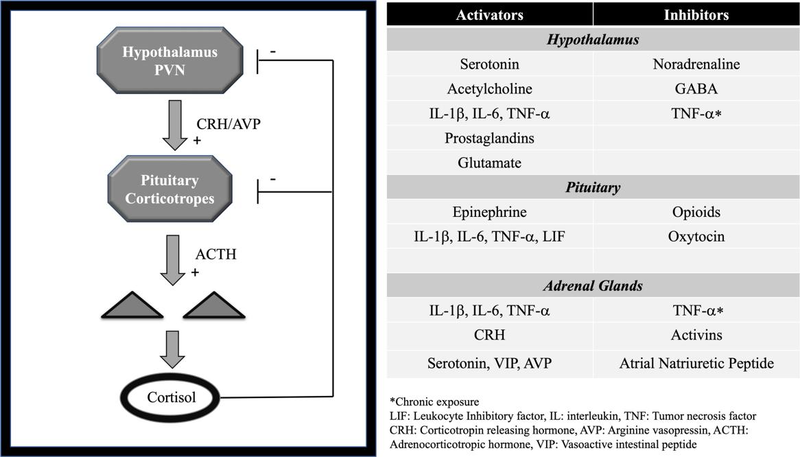
Figure 1. The hypothalamic-pituitary-adrenal axis regulation [24–27].
Cortisol‘s actions are further regulated by enzymatic transformation of its active and inactive forms. In the kidney, colon, salivary glands, and placenta, 11-β-hydroxysteroid dehydrogenase type 2 inactivates cortisol into cortisone, protecting these tissues from overstimulation of the mineralocorticoid receptor. In most other tissues, but mainly in the liver, 11-β-hydroxysteroid dehydrogenase type 1 converts cortisone into cortisol, augmenting the GC effects when needed [28].
皮质醇的作用通过酶对其活性和非活性形式的转化进一步调节。在肾脏、结肠、唾液腺和胎盘中,11-β-羟基类固醇脱氢酶2型可使皮质醇失活,转化为可的松,保护这些组织免受矿化皮质激素受体的过度刺激。在大多数其他组织中,但主要在肝脏中,11-β-羟基类固醇脱氢酶1型将可的松转化为皮质醇,在需要时增强GC作用[28]。
Multiple factors determine the degree of HPA axis activation during a surgical procedure. Individual factors, including genetics, age, sex, comorbidities, and medications, as well as perioperative factors, such as type and duration of anesthesia and operative procedure and perioperative complications, contribute to the heterogeneity seen in studies evaluating the HPA axis response [29].
多种因素决定了手术过程中下丘脑轴的激活程度。个体因素,包括遗传、年龄、性别、合并症和药物,以及围手术期因素,如麻醉类型和持续时间、手术方式和围手术期并发症,导致了评估HPA轴反应的研究中出现的异质性[29]。
Different types of surgical procedures generate different degrees of HPA axis activation [29–31]. Criteria to stratify the degree of surgical risk (Grade I-III), independent from the anesthetic risk, are summarized in Table 1 [32]. Multiple studies including a systematic review and meta-analysis of 71 studies including 2953 patients show that cortisol levels increase in proportion to the grade of surgery [29–31, 33, 34]. In Grade I procedures, no intraoperative cortisol peak is observed, whereas in Grade II procedures, peak cortisol occurs at the time of extubation and returns to baseline at 24 hours [29, 30]. With Grade III procedures, peak cortisol occurs 6 to 18 hours postoperatively, persists for 24 hours, and returns to baseline by postoperative day 5 to 7 [29–31, 33, 34]. Therefore, the maximum activation of the HPA axis occurs within the first 24 hour postoperatively, returning to baseline in <7 days even in patients undergoing Grade III operations [35, 36].
32. Donati A, Ruzzi M, Adrario E, et al.. A new and feasible model for predicting operative risk. Br J Anaesth. 2004;93(3):393–399. doi: 10.1093/bja/aeh210 [PubMed] [CrossRef] [Google Scholar]
不同类型的手术产生不同程度的HPA轴激活[29-31]。表1总结了独立于麻醉风险的手术风险分级标准(I-III级)[32]。包括对71项研究(包括2953例患者)的系统回顾和荟萃分析在内的多项研究表明,皮质醇水平随手术级别的增加而增加[29 - 31,33,34]。在I级手术中,未观察到术中皮质醇峰值,而在II级手术中,皮质醇峰值出现在拔管时,并在24小时内恢复到基线[29,30]。在III级手术中,皮质醇峰值出现在术后6 - 18小时,持续24小时,并在术后第5 - 7天恢复至基线[29 - 31,33,34]。因此,HPA轴的最大激活发生在术后第一个24小时内,即使在III级手术的患者中,也在<7天内恢复到基线[35,36]。
Table 1.Surgical stress associated with common surgical procedures, based on the modified Johns Hopkins surgical criteria [ 32 ]
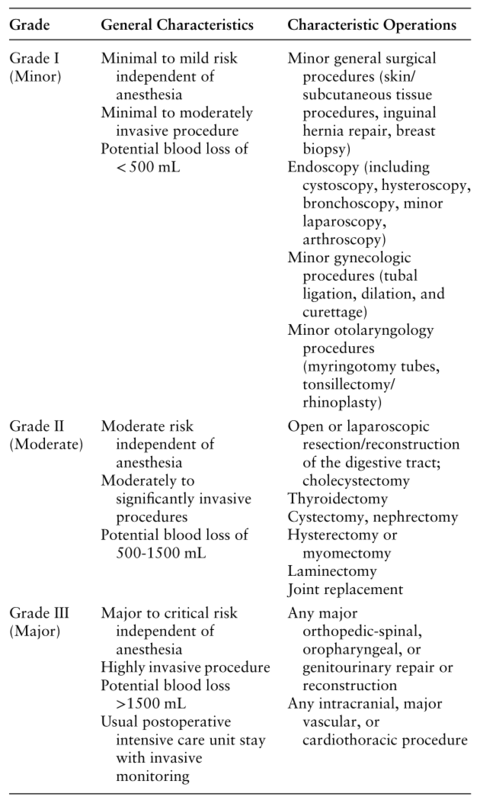
Grade General Characteristics Characteristic Operations
Grade I
(Minor) Minimal to mild risk independent of anesthesia
Minimal to moderately invasive procedure
Potential blood loss of < 500 mL Minor general surgical procedures (skin/subcutaneous tissue procedures, inguinal hernia repair, breast biopsy)
Endoscopy (including cystoscopy, hysteroscopy, bronchoscopy, minor laparoscopy, arthroscopy)
Minor gynecologic procedures (tubal ligation, dilation, and curettage)
Minor otolaryngology procedures (myringotomy tubes, tonsillectomy/rhinoplasty)
Grade II
(Moderate) Moderate risk independent of anesthesia
Moderately to significantly invasive procedures
Potential blood loss of 500-1500 mL Open or laparoscopic resection/reconstruction of the digestive tract; cholecystectomy
Thyroidectomy
Cystectomy, nephrectomy
Hysterectomy or myomectomy
Laminectomy
Joint replacement
Grade III
(Major) Major to critical risk independent of anesthesia
Highly invasive procedure
Potential blood loss >1500 mL
Usual postoperative intensive care unit stay with invasive monitoring Any major orthopedic-spinal, oropharyngeal, or genitourinary repair or reconstruction
Any intracranial, major vascular, or cardiothoracic procedure
To understand the perioperative HPA axis response, Udelsman et al measured blood samples every 10 minutes during and after Grade II neck operations performed with identical sedation and anesthetic regimens. Interestingly, CRH(Corticotropin-Releasing Hormone)促肾上腺皮质激素释放激素, ACTH(Adrenocorticotropic Hormone)促肾上腺皮质激素, and cortisol levels were unchanged intraoperatively. However, there was a marked elevation in ACTH and cortisol levels during anesthetic reversal, endotracheal extubation, and immediately postoperatively [37]. In contrast, in Grade III operations, cortisol remained elevated on postoperative day 2, but there was a marked reduction in CRH and ACTH levels [38]. A recently reported significant decrease in cortisol clearance during critical illness could explain this postoperative day 2 dissociation between CRH/ACTH and cortisol levels through negative feedback [39]. Furthermore, neural regulation of the HPA axis seem to have an important role in the modulating the stress response to surgical procedures. Studies showing that epidural or local anesthesia can interrupt HPA axis activation following a surgical incision highlight its role [40, 41]. Also, the sympathetic nerves of the adrenal cortex can facilitate the ACTH response [42].
为了了解围手术期HPA轴反应,Udelsman等人在采用相同镇静和麻醉方案的II级颈部手术期间和手术后每10分钟测量一次血液样本。有趣的是,CRH、ACTH和皮质醇水平在术中没有变化。然而,在麻醉逆转、气管内拔管和术后立即出现ACTH和皮质醇水平明显升高[37]。相比之下,在III级手术中,皮质醇在术后第2天仍然升高,但CRH和ACTH水平明显降低[38]。最近报道的危重疾病期间皮质醇清除率的显著下降可以解释术后第2天CRH/ACTH和皮质醇水平通过负反馈分离的原因[39]。此外,下丘脑轴的神经调节似乎在外科手术应激反应的调节中起着重要作用。研究表明,硬膜外麻醉或局部麻醉可以阻断手术切口后HPA轴的激活,突出了其作用[40,41]。肾上腺皮质交感神经也可促进ACTH反应[42]。
In addition to an increase in cortisol secretion, immediately postoperatively there is an approximately 30% to 50% decrease in CBG(centromere ba giemsaband)皮质类固醇结合球蛋白, and this reduction persists at 24 hours after surgery, resulting in elevated free cortisol levels [43, 44]. This increment in free cortisol could be associated with inflammation, given that interleukin-6 decreases CBG concentration by about half and the affinity of CBG for cortisol is reduced by neutrophil activation and fever, both common perioperatively [18, 45].
除了皮质醇分泌增加外,术后CBG(centromere ba giemsaband)皮质类固醇结合球蛋白立即下降约30%至50%,并且这种下降持续到术后24小时,导致游离皮质醇水平升高[43,44]。游离皮质醇的增加可能与炎症有关,因为白细胞介素-6会使CBG浓度降低约一半,而CBG对皮质醇的亲和力会因中性粒细胞激活和发热而降低,这两种情况在围手术期都很常见[18,45]。
Perioperative Symptoms of Adrenal Insufficiency and Cardiovascular Collapse
GCs have an essential role in the regulation of vascular tone, cardiac, and adrenomedullary function. In blood vessels, they have a permissive role in the action of vasoactive substances, particularly catecholamines [46, 47]. Additionally, GCs promote vasoconstriction by inhibiting endothelial production of nitric oxide and prostacyclin [17, 47], upregulating angiotensin II receptor AT1 [48] as well as increasing α-1 adrenergic receptors and norepinephrine-binding affinity in the vascular smooth muscle cells [47, 49]. Accordingly, during an adrenal crisis, there is a markedly reduced vascular tone resulting in hypotension that can eventually become refractory to vasopressors.
GCs在调节血管张力、心脏和肾上腺髓质功能方面起着重要作用。在血管中,它们对血管活性物质,特别是儿茶酚胺的作用具有permissive作用[46,47]。此外,GCs通过抑制内皮细胞一氧化氮和前列环素的生成[17,47],上调血管紧张素II受体AT1[48],以及增加血管平滑肌细胞中α-1肾上腺素能受体和去甲肾上腺素结合亲和力来促进血管收缩[47,49]。因此,在肾上腺危象期间,血管张力明显降低,导致低血压,最终对血管加压药物产生难治性反应。
In the heart, GCs increase contractility by increasing the expression of genes that regulate intracellular calcium concentrations [50]. Studies in AI reported reduced stroke volume and cardiac index with increased systemic arterial resistance independent of changes in serum sodium and potassium levels [51, 52]. Additionally, AI has been associated with high-output heart failure that can resemble the septic shock-associated loss of vascular tone [52, 53]. These 2 hemodynamic profiles associated with hypotension depends on the degree of fluid resuscitation and the time the right heart catheterization is performed [52]. Patients with AI may have prolongation of the PR interval, which can reach a first-degree AV block or worsen the underlying AV conduction abnormalities [54]. These effects on cardiac output and the conduction system in AI can be reversed by GC replacement [55–57].
在心脏中,GCs通过增加调节细胞内钙浓度的基因表达来增加收缩力[50]。AI的研究报告称,与血清钠和钾水平的变化无关,卒中容量和心脏指数减少,全身动脉阻力增加[51,52]。此外,AI与高输出量心力衰竭有关,类似于感染性休克相关的血管张力丧失[52,53]。这两种与低血压相关的血流动力学特征取决于液体复苏的程度和右心导管插入的时间[52]。AI患者可延长PR间期,可达到一级房室传导阻滞或加重潜在房室传导异常[54]。这些对AI的心输出量和传导系统的影响可以通过GC替代来逆转[55-57]。
Adrenal medullary development and function are highly dependent on the presence of adequate amounts of cortisol [58, 59]. The expression and activity of phenylethanolymine N-methyltransferase, the enzyme that converts norepinephrine into epinephrine, requires a high intra-adrenal cortisol concentration that is maintained by the adrenoportal system between the cortex and the medulla [60]. Glucocorticoids also inhibit catechol-O-methyl-transferase, resulting in decreased clearance of epinephrine [47]. Therefore, the adrenomedullary function can become severely compromised in AI with markedly low levels of epinephrine at baseline and incomplete response during stress [61].
肾上腺髓质的发育和功能高度依赖于足量皮质醇的存在[58,59]。苯乙醇胺n -甲基转移酶是将去甲肾上腺素转化为肾上腺素的酶,其表达和活性需要皮质和髓质的肾上腺门静脉系统维持较高的肾上腺内皮质醇浓度[60]。糖皮质激素还会抑制儿茶酚- o -甲基转移酶,导致肾上腺素清除率降低[47]。因此,在AI中,肾上腺素基线水平明显降低,应激反应不完全,肾上腺髓功能可能严重受损[61]。
Beyond cardiovascular instability and collapse, the symptoms of AI in the perioperative period can be subtle and may be missed due to their similarity to common postoperative complaints including anorexia, fatigue, nausea, vomiting, abdominal pain, muscle cramps, weakness, dizziness, and lethargy [62]. The clinical team must be aware of GC withdrawal symptoms that may occur in patients on chronic GC on a fast perioperative taper; these patients who may experience AI-type symptoms despite being maintained on supraphysiological GC doses [63, 64].
除了心血管不稳定和塌陷外,围手术期AI的症状可能很微妙,可能被忽略,因为它们与术后常见的症状相似,包括厌食、疲劳、恶心、呕吐、腹痛、肌肉痉挛、虚弱、头晕和嗜睡[62]。临床团队必须意识到在围手术期快速减少的慢性GC患者中可能出现的GC戒断症状;这些患者尽管维持了生理上的GC剂量,但仍可能出现AI型症状[63,64]。
The HPA Axis in Patients Using Glucocorticoids
Pharmacological Properties of Glucocorticoids
Glucocorticoids have historically been classified as short, intermediate, and long-acting according to their biological half-life (Table 2). However, GCs have many other variable pharmacologic properties, including administration route, potency, and affinity for the GC receptor, resulting in a heterogeneous group of drugs with different potentials to suppress the HPA axis.
Table 2.Pharmacological properties of frequently used glucocorticoids [ 65–68 ]
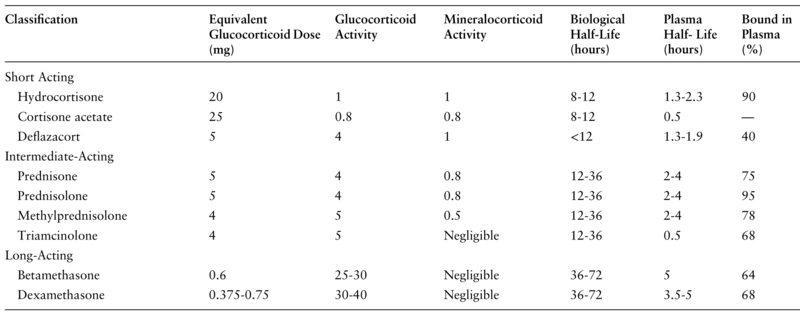
Classification Equivalent Glucocorticoid Dose (mg) Glucocorticoid Activity Mineralocorticoid Activity Biological Half-Life (hours) Plasma Half- Life (hours) Bound in Plasma (%)
Short Acting
Hydrocortisone 20 1 1 8-12 1.3-2.3 90
Cortisone acetate 25 0.8 0.8 8-12 0.5 —
Deflazacort 5 4 1 <12 1.3-1.9 40
Intermediate-Acting
Prednisone 5 4 0.8 12-36 2-4 75
Prednisolone 5 4 0.8 12-36 2-4 95
Methylprednisolone 4 5 0.5 12-36 2-4 78
Triamcinolone 4 5 Negligible 12-36 0.5 68
Long-Acting
Betamethasone 0.6 25-30 Negligible 36-72 5 64
Dexamethasone 0.375-0.75 30-40 Negligible 36-72 3.5-5 68
In general, the absorption rate after oral administration is rapid (30-45 minutes) and similar among different preparations with bioavailability ranging from 60% to almost 100% [69,70]. Intramuscular absorption is rapid, whereas the absorption after subcutaneous injections is slightly slower depending on the amount of adipose tissue present [71]. Systemic absorption from other formulations such as inhaled, topical, ophthalmic, buccal, or rectal administration is lower, but all can potentially suppress the HPA axis [72]. Topical and mucosal absorption of GCs depends on the integrity of the skin/epithelial barrier, which is modified by inflammation and influenced by the thickness of skin [72]. Certain GCs such as Triamcinolone after intra-articular and epidural injection are slowly absorbed resulting in sustained supraphysiological concentration. Oral budesonide has a high first-pass effect in the liver where about 90% is inactivated. Despite this, HPA axis suppression and cases of adrenal crises have been reported [73, 74].
一般来说,口服给药后的吸收速度很快(30-45分钟),不同制剂的吸收速度相似,生物利用度从60%到几乎100%不等[69,70]。肌内吸收迅速,而皮下注射后的吸收略慢,这取决于脂肪组织的数量[71]。其他剂型(如吸入、外用、眼用、口用或直肠给药)的全身吸收较低,但都可能抑制HPA轴[72]。GCs的局部和粘膜吸收取决于皮肤/上皮屏障的完整性,而皮肤/上皮屏障会受到炎症和皮肤厚度的影响[72]。某些GCs如曲氨奈德在关节内和硬膜外注射后被缓慢吸收,导致持续的生理上浓度升高。口服布地奈德在肝脏中有很高的首过效应,其中约90%是失活的。尽管如此,HPA轴抑制和肾上腺危象的病例仍有报道[73,74]。
Incidence of Postoperative Adrenal Insufficiency
Postoperative AI in patients on chronic GCs is rare [8, 17, 75]. However, establishing the true incidence is challenging. In many cases, hypotension that resolves in response to GCs, in the absence of an alternative explanation, has been used as the criteria to establish the diagnosis of AI. A 1994 review of 57 cases of patients on chronic GCs who died possibly from postoperative adrenal crisis found confirmation in only 3 cases [8]; this and other reports [33, 76] indicate that post-op AI may not be as common as initially thought. In some instances, alternative etiologies such as major blood loss, anaphylaxis, or sepsis could have explained the postoperative hypotension. However, the incidence of postoperative AI may be underreported since reporting such statistics may be undesirable for hospitals. There is a clear need for further studies in this subject.
慢性GCs患者术后AI罕见[8,17,75]。然而,确定真正的发病率是具有挑战性的。在许多病例中,在没有其他解释的情况下,低血压因GCs而消退,已被用作确定AI诊断的标准。1994年对57例可能死于术后肾上腺危机的慢性GCs患者的回顾发现,只有3例得到证实[8];这一研究和其他报告[33,76]表明,术后AI可能并不像最初想象的那样普遍。在某些情况下,其他病因如大量失血、过敏反应或败血症可以解释术后低血压。然而,术后AI的发生率可能被低估,因为报告这样的统计数据对医院来说可能是不可取的。这一课题显然需要进一步的研究。
Risk for HPA Axis Suppression in Different Clinical Scenarios
The risk for HPA axis suppression in relation to GC exposure depends on multiple factors including the type of GC, ability of the drug to reach the systemic circulation, dose, and duration of treatment. However, the exact dose and duration that results in clinically significant AI has been a matter of debate [64, 77–80]. In general, systemic treatments, long-acting GCs, higher doses, longer duration of therapy, higher potency, nighttime administration, multiple cycles of treatment, and multiple daily doses carry a higher risk for HPA axis suppression and AI [64, 77–82]. Cushingoid features should alert clinicians of the presence of HPA suppression. Stopping chronic GCs close to an operation may have a significant impact on the risk for postoperative AI. Regardless, there is a poor correlation between biochemical HPA axis suppression and clinical outcome (AI) [77–80, 83, 84].
与GC暴露相关的HPA轴抑制风险取决于多种因素,包括GC类型、药物到达体循环的能力、剂量和治疗时间。然而,导致临床显著AI的确切剂量和持续时间一直存在争议[64,77 - 80]。一般来说,全身治疗、长效GCs、高剂量、长时间治疗、高效力、夜间给药、多周期治疗和每日多次给药会增加HPA轴抑制和AI的风险[64,77 - 82]。库欣样特征应提醒临床医生HPA抑制的存在。手术前停止慢性GCs可能会对术后AI的风险产生重大影响。无论如何,生化HPA轴抑制与临床结果(AI)相关性较差[77 - 80,83,84]。
Oral glucocorticoids
The daily requirement of a patient with AI to maintain basal, nonstressed body functions is approximately 4 to 5 mg prednisone equivalent per day. To stratify the risk of HPA axis suppression, GC intake may be divided into low dose (<5 mg prednisone equivalent), medium dose (5-20 mg), and high dose (>20 mg) per day. The duration of GC exposure can be considered as short-term (<1 month), intermediate-duration (between 1-3 months), and long-term (>3 months). However, these subclassifications have a role in perioperative management of patients if there is a plan to stop GCs before the procedure. Otherwise, most patients should receive a short course of GCs perioperatively based on the level of surgical stress and then return to their basal dose as they recover.
AI患者维持基础、非应激身体功能的每日需要量约为每天4至5mg强的松当量。为了对HPA轴抑制的风险进行分层,GC摄入可分为低剂量(强的松当量< 5mg)、中剂量(5- 20mg)和高剂量(> 20mg)每天。GC暴露时间可分为短期(<1个月)、中期(1-3个月)和长期(>3个月)。然而,如果在手术前有停止GCs的计划,这些亚分类在患者的围手术期管理中具有作用。否则,大多数患者应根据手术应激水平在围手术期接受短疗程的GCs,然后在恢复后返回其基础剂量。
Low-dose GCs administered in the morning, for any duration of time, seem to have a low risk of causing clinically significant AI. In a study of 50 patients on long-term prednisone (mean duration approximately 4 years), those receiving <5 mg/day showed a normal or near-normal response to the Cosyntropin stimulation test (CST) without AI-related events [85]. Accordingly, we do not recommend additional workup in patients taking <5 mg prednisone equivalent per day in the morning beyond the continuation of their glucocorticoids and monitoring them for any signs and symptoms of AI. However, it is also important to consider the cumulative effect of previous glucocorticoids in patients in whom the dose of GCs has been tapered to less than 5 mg prednisone equivalent per day at the time of their surgical evaluation. Such patients should receive a short course of parenteral GC therapy when unable to take their daily GC dose.
在早晨给予低剂量GCs,在任何时间内,似乎具有低风险引起临床显著的AI。在一项对50名长期使用强的松(平均持续时间约为4年)的患者的研究中,那些接受< 5mg /天的患者Cosyntropin刺激试验(CST)的反应正常或接近正常,没有AI相关事件[85]。因此,除了继续使用糖皮质激素和监测AI的任何体征和症状外,我们不建议每天早晨服用<5 mg强的松当量的患者进行额外的检查。然而,在手术评估时,当GCs剂量逐渐减少到每天少于5mg泼尼松当量时,考虑既往糖皮质激素的累积效应也很重要。当不能服用每日GC剂量时,此类患者应接受短期肠外GC治疗。
High-dose GCs taken for short-term frequently cause HPA axis suppression although rarely clinically significant AI. Carella et al reported that a short course of high dose of prednisone (40 mg 3 times per day for 3 days followed by a taper during the subsequent 4 days) resulted in transient HPA axis suppression [86]. HPA axis recovery based on a CRH and CST was seen 1 week after treatment. Similarly, Spiegel et al showed that prednisone at doses of 40 mg/m2 to 100 mg/m2 daily for <1 month could result in HPA axis suppression with recovery in <1 week and no clinically significant AI events. In the authors’ experience, it is common to see random and morning cortisol levels <5 µg/dL within a few days after a 6-day course of methylprednisolone (“Medrol pack”) or short courses of “pulse doses” of methylprednisolone (250-1000 mg). However, neither the literature review nor our observations have found clinically significant AI-related events in such patients. Therefore, the duration of therapy seems to have a greater impact than the dose on HPA axis suppression. Indeed, all historically reported adrenal crises have been in patients on chronic GC therapy. Therefore, we do not recommend any action outside of routine perioperative monitoring in patients who have been on GCs for <4 weeks prior to surgery.
短期服用大剂量GCs经常引起HPA轴抑制,但很少有临床意义的AI。Carella等人报道,高剂量强的松短期疗程(40mg /天,3次,连续3天,随后4天逐渐减少)可导致短暂的HPA轴抑制[86]。治疗1周后观察基于CRH和CST的HPA轴恢复情况。同样,Spiegel等人表明,强的松剂量为每天40 mg/m2至100 mg/m2,持续<1个月,可导致HPA轴抑制,并在<1周内恢复,无临床显著的AI事件。根据作者的经验,在6天甲基强的松龙疗程(“Medrol pack”)或短期“脉冲剂量”甲基强的松龙疗程(250-1000 mg)后的几天内,常见的是随机和早晨皮质醇水平<5µg/dL。然而,无论是文献综述还是我们的观察,都没有在这些患者中发现具有临床意义的AI相关事件。因此,治疗时间似乎比剂量对HPA轴抑制的影响更大。事实上,所有历史上报道的adrenal crises都发生在慢性GC治疗的患者身上。因此,我们不建议术前服用GCs <4周的患者在常规围手术期监测之外采取任何行动。
Patients taking 5 mg or more of prednisone equivalent per day for >1 month may have variable degrees of HPA axis suppression [87]. The risk of HPA axis suppression is higher in patients taking higher doses for a longer duration. Such patients should not stop GCs prior to surgery without further evaluation of the HPA axis. The majority of these patients can stay on their current dose of GC perioperatively and can be administered a short course of intravenous GC postoperatively until they resume their oral intake (see therapy section).
每天服用5mg或以上强的松当量,持续>1个月的患者可能出现不同程度的HPA轴抑制[87]。服用高剂量、持续时间较长的患者HPA轴抑制的风险较高。此类患者在手术前不应在未对下丘脑轴进行进一步评估前停止GCs治疗。这些患者中的大多数可以在围手术期保持当前剂量的GC,并可以在术后给予短疗程的静脉GC,直到他们恢复口服摄入(见治疗部分)。
Other variables that influence the risk of HPA axis suppression The time of the day of GC administration can influence the risk of HPA axis suppression. In 8 healthy subjects who received 0.5 mg of dexamethasone at midnight for 2 consecutive nights, there was a more prolonged HPA axis suppression compared to those who received the same regimen at 8 am or 4 pm [88]. The administration of multiple GC doses throughout the day carries a higher risk for HPA axis suppression than the same total daily dose taken once a day [87, 89]. Myles et al in a crossover design compared cortisol levels after giving patients a single dose of prednisolone at 10 am for 8 weeks vs the same total daily dose divided as half at 10 am and the other half at 10 pm for 8 weeks, returning to a single daily dose after [89]. Cortisol levels were significantly lower in patients during the time the dose was split compared to when patients received a single daily dose of prednisolone. Alternate-day (morning) GC regimens are associated with a lower incidence of iatrogenic Cushing syndrome and HPA axis suppression [64, 90].
影响HPA轴抑制风险的其他变量GC给药的时间可以影响HPA轴抑制的风险。在8名健康受试者中,连续2晚在午夜接受0.5 mg地塞米松治疗,与在上午8点或下午4点接受相同治疗的受试者相比,HPA轴抑制时间更长[88]。全天多次给药GC比每天一次总给药GC有更高的HPA轴抑制风险[87,89]。Myles等人在交叉设计中比较了在上午10点给予患者单剂量强的松龙并持续8周后的皮质醇水平与相同的每日总剂量,即上午10点一半,晚上10点一半,持续8周,之后恢复到每日单剂量[89]。与患者接受单剂量泼尼松龙相比,患者在剂量分开期间的皮质醇水平显着降低。隔天(早晨)GC方案与较低的医源性库欣综合征和HPA轴抑制发生率相关[64,90]。
There is a higher risk of AI-related clinical events if GCs are stopped closer to the date of surgical procedure, as the HPA axis needs time to recover [34]. In patients with inflammatory bowel disease (IBD) taking up to 180 mg/day of prednisone for 2 to 23 months, the ones who stopped prednisone >2 months prior to surgery had the lowest risk of developing AI-related hypotension [76]. This retrospective study should be interpreted with caution since, when adjusted by disease severity, no differences in hypotension were observed.
如果离手术日期更近的时候停止GCs,发生AI相关临床事件的风险更高,因为下丘脑轴需要时间恢复[34]。在炎症性肠病(IBD)患者中,连续2 ~ 23个月服用180 mg/天强的松的患者,术前>2个月停用强的松的患者发生AI相关性低血压的风险最低[76]。这项回顾性研究应谨慎解释,因为当按疾病严重程度调整时,没有观察到低血压的差异。
Nonoral glucocorticoid use
Intra-articular and epidural injections
The risk of AI with intra-articular GCs remains largely unrecognized and likely underestimated [72]. Both the absorption and the ability to suppress the HPA axis are proportional to the GC dose, half-life, solubility, vascularization of the synovium (increased during inflammation), number of joints injected, and frequency of injections [91–93]. In a meta-analysis, the intra-articular and the oral route carried the highest risk for HPA axis suppression [81]. In a small randomized controlled study that used a single fixed dose of 80 mg of methylprednisolone knee injection vs placebo, 25% of the patients receiving GC developed HPA axis suppression between week 2 and 4 after injection, and then returned to baseline [94]. When multiple joints are simultaneously injected, HPA axis suppression takes longer to recover. Habib et al reported that injecting 80 mg of methylprednisolone acetate in both knees simultaneously resulted in HPA axis suppression in 60% of the patients by week 1, 30% to 35% between week 2 and 4, and 10% of patients at week 8 [95]. Epidural GC injections can also result in rapid and prolonged HPA axis suppression. A single dose of triamcinolone resulted in suppression of the HPA axis within 1 week, slowly returning to baseline in about 4 to 12 weeks [96]. We receive a substantial number of referrals for AI of unknown etiology where previous GC injections were not elicited from the history. Until better quality data is available, we recommend evaluating the HPA axis in patients who have received 3 or more GC injections within 6 months before surgery [97].
AI伴关节内GCs的风险在很大程度上仍未被认识,而且可能被低估了[72]。吸收和抑制HPA轴的能力都与GC剂量、半衰期、溶解度、滑膜血管化(炎症时增加)、注射关节数和注射频率成正比[91-93]。在一项荟萃分析中,关节内和口腔途径对HPA轴抑制的风险最高[81]。在一项小型随机对照研究中,使用单次固定剂量80mg甲基强的松龙膝关节注射与安慰剂,25%接受GC的患者在注射后第2至4周出现HPA轴抑制,然后恢复到基线[94]。当多个关节同时注射时,HPA轴的抑制需要更长的时间才能恢复。Habib等报道,双膝同时注射80mg醋酸甲基强的松龙导致60%的患者在第1周,30%至35%的患者在第2周至第4周,10%的患者在第8周抑制HPA轴[95]。硬膜外GC注射也可导致HPA轴的快速和长时间抑制。单剂量曲安奈德可在1周内抑制HPA轴,约4 - 12周后缓慢恢复至基线[96]。我们收到大量的转介不明病因的AI,以前的GC注射不是从历史中引出的。在获得更好质量的数据之前,我们建议在手术前6个月内接受过3次或更多GC注射的患者中评估HPA轴[97]。
Inhaled and intranasal
Inhaled GCs are absorbed systemically by the pulmonary vasculature with a smaller fraction swallowed and absorbed by the gastrointestinal tract [72]. In a systematic review in patients with asthma using only inhaled GCs, higher risk of HPA axis suppression was seen with high doses of fluticasone equivalent (>1000 mcg/day), compared to medium (200-1000 mcg/day) and low doses (<200 mcg/day), resulting in AI in 18.5%, 5.4% and 1.5% of patients, respectively. When analyzed by the duration of treatment, long-term use (>1 year) had a higher prevalence of AI compared to medium (1 month to 1 year) and short term (<1 month); duration of treatment was associated with abnormal HPA axis in 20.3%, 9.0%, and 1.3% of patients, respectively [81]. Another meta-analysis showed that the risk of basal cortisol suppression was higher with fluticasone compared to other inhaled steroids at the same equivalent dose [98]. The literature on AI from intranasal GCs is scarce. Intranasal fluticasone preparations in the United States are sold in quantities of 27.5 mcg and 50 mcg per spray. However, fluticasone nasal sprays purchased outside the United States may have higher strengths; authors have treated a patient who purchased fluticasone abroad at a strength of 250 mcg per spray resulting in GC-induced AI.
吸入的GCs通过肺血管系统吸收,少部分被胃肠道吞食吸收[72]。在一项仅使用吸入性GCs的哮喘患者的系统评价中,与中剂量(200-1000 mcg/天)和低剂量(<200 mcg/天)相比,高剂量氟替卡松当量(>1000 mcg/天)的HPA轴抑制风险更高,分别导致18.5%、5.4%和1.5%的患者发生AI。按治疗时间分析,长期用药(>1年)的AI患病率高于中期用药(1个月至1年)和短期用药(<1个月);治疗时间与HPA轴异常相关的患者分别为20.3%、9.0%和1.3%[81]。另一项荟萃分析显示,在相同当量剂量下,氟替卡松与其他吸入类固醇相比,基底皮质醇抑制的风险更高[98]。关于鼻内GCs AI的文献很少。在美国,鼻用氟替卡松制剂的销售数量分别为27.5微克和50微克。然而,在美国以外购买的氟替卡松鼻喷雾剂可能具有更高的强度;作者治疗了一名从国外购买氟替卡松的患者,每剂剂量为250微克,导致GC-induced的AI。
Topical glucocorticoids
Topical GCs are classified based on potency into 7 classes and 3 subgroups: ultra-high (Class 1-3), moderate (Class 4-5), and low (Class 6-7) potency corticosteroids. Topical GCs can be absorbed systemically through the skin depending on the surface area of application, location applied, and skin thickness and integrity of the skin (ie, ulcerated, injured, or inflamed skin results in greater absorption) [72]. Suppression of the HPA axis and AI can occur with the most potent topical GCs, clobetasol propionate 0.05% (Class 1), and betamethasone dipropionate 0.05% (Class 2). Iatrogenic Cushing syndrome accompanied by HPA axis suppression has been reported in adults with doses of approximately 33 to 100 g/week of clobetasol propionate and 49 to 80 mg/week of beclomethasone dipropionate [99–101]. A fingertip of GC in a 5 mm nozzle tube is about 0.5 g (slightly lower in women) [102]. HPA axis suppression may appear within days of the use of high potency topical GC and the duration of suppression is variable from weeks to months [99–101]. A 2002 literature review identified 1 fatality due AI in a child on whom large amounts of topical betamethasone was applied for generalized psoriasis [103]. Therefore, patients using potent topical GCs for prolonged periods of time or in combination with systemic GCs need to be cautious about the development of AI.
外用皮质类固醇根据效力分为7类和3个亚组:超高(1-3类)、中等(4-5类)和低(6-7类)效力皮质类固醇。局部GCs可以通过皮肤被全身吸收,这取决于应用的表面积、应用的位置、皮肤厚度和皮肤的完整性(即溃疡、受伤或发炎的皮肤会导致更大的吸收)[72]。最有效的外用GCs, 0.05%丙酸氯倍他索(1类)和0.05%二丙酸倍他米松(2类)可抑制HPA轴和AI。据报道,在成人中,剂量约为33至100g /周的丙酸氯倍他索和49至80mg /周的二丙酸倍他索可导致HPA轴抑制的医源性库欣综合征[99-101]。在5mm的喷嘴管中,指尖的气相色谱约为0.5 g(女性略低)[102]。HPA轴抑制可在使用高效局部GC后数天内出现,抑制持续时间从数周到数月不等[99-101]。2002年的一篇文献综述发现,1例儿童因广泛性牛皮癣大量外用倍他米松而致AI死亡[103]。因此,长期使用强效局部GCs或与全身GCs联合使用的患者需要谨慎对待AI的发展。
Other routes
The ophthalmic and rectal routes of steroid application have been associated with HPA axis suppression in small studies or case reports. Ophthalmic solutions, when used excessively and for prolonged periods, can be systemically absorbed resulting in iatrogenic Cushing syndrome and HPA axis suppression [104]. Rectal GCs (enemas) have been associated with systemic absorption and HPA axis suppression particularly when the rectal mucosa is injured, inflamed, or ulcerated [105].
在小型研究或病例报告中,眼科和直肠类固醇应用途径与HPA轴抑制有关。当长期过量使用眼液时,可被全身吸收,导致医源性库欣综合征和HPA轴抑制[104]。直肠GCs(灌肠)与全身吸收和HPA轴抑制有关,特别是当直肠粘膜受损、发炎或溃烂时[105]。
Special Situations
Many GCs are metabolized via cytochrome P450 3A4 (CYP3A4). Therefore, inhibition of this enzyme results in risk of higher GC levels and HPA axis suppression. Multiple medications are known to inhibit CYP3A4; for example, ritonavir, a protease inhibitor, has a known interaction with inhaled fluticasone [92, 106, 107]. When taken concomitantly with ritonavir, fluticasone propionate at doses as low as 500 ug/day for 2 months can result in iatrogenic Cushing syndrome [108]. Cobicistat, an HIV drug enhancer which also inhibits CYP3A4 when used with ritonavir, can make this interaction more severe [109]. Beclomethasone dipropionate is metabolized to a lesser degree by CYP3A4 and therefore could be an alternative to fluticasone when a combination with CYP3A4 inhibitors is required. Conversely, when CYP3A4 inducers are used, GCs metabolism increases, which may result in increased risk of perioperative AI in patients on a fixed GC dose. Some examples of CYP3A4 inducers include antiepileptic drugs, rifampin, and mitotane [92]. A list of drugs affecting the HPA axis is included in Table 3 [92, 110, 111].
许多GCs通过细胞色素P450 3A4 (CYP3A4)代谢。因此,抑制这种酶会导致GC水平升高和HPA轴抑制的风险。已知多种药物可抑制CYP3A4;例如,蛋白酶抑制剂利托那韦(ritonavir)已知与吸入的氟替卡松有相互作用[92,106,107]。当与利托那韦同时服用时,低至500微克/天的丙酸氟替卡松连续2个月可导致医源性库欣综合征[108]。Cobicistat是一种HIV药物增强剂,与利托那韦一起使用时也会抑制CYP3A4,可使这种相互作用更加严重[109]。二丙酸倍氯米松被CYP3A4代谢的程度较低,因此当需要与CYP3A4抑制剂联合使用时,可作为氟替卡松的替代品。相反,当使用CYP3A4诱导剂时,GC代谢增加,这可能导致固定GC剂量患者围手术期AI的风险增加。一些CYP3A4诱导剂的例子包括抗癫痫药、利福平和米托坦[92]。影响下丘脑轴的药物列表见表3[92,110,111]。
Table 3. Common drugs affecting the hypothalamic-pituitary adrenal axis [ 92, 97, 110, 111]

Drugs Affecting CRH and ACTH Synthesis or Secretion
Opioids (morphine, oxycodone, fentanyl, tramadol, methadone, heroin)
Benzodiazepines (midazolam, oxazepam, alprazolam, diazepam)
Megestrol Acetate
Medroxyprogesterone
Drugs Affecting Cortisol Synthesis
Azoles (Ketoconazole/Levoketoconazole, Posaconazole, fluconazole)
Etomidate
Metyrapone, Aminoglutethimide
Trilostane
Drugs Affecting Cortisol Action
Mifepristone
Drugs Affecting Cortisol Metabolism
Cytochrome P450 3A4 Inhibitors
(Decrease Cortisol Metabolism) Cytochrome P450 3A4 Inducers
(Increase Cortisol Metabolism)
Protease inhibitors (darunavir, indinavir, lopinavir, nelfinavir, telaprevir, ritonavir) Antiepileptic drugs (phenytoin, fosphenytoin, phenobarbital, primidone)
Cobicistat, Azoles (itraconazole, voriconazole), clarithromycin, Grapefruit juice, mifepristone Carbamazepine, rifampin, and mitotane
Calcium channel blockers (verapamil, diltiazem), amiodarone, cimetidine, conivaptan, erythromycin, and imatinibb Nafcillin, rifabutin, bosentan, dorafenib, efavirenz, rifabutin, St John‘s worta
Open in a separate window
a Less potent inducers.
b Less potent inhibitors.
Opioids such as morphine, oxycodone, fentanyl, tramadol, methadone, and heroin have been increasingly recognized as cause of GC-induced AI [112]. Li et al reported a prevalence of about 9% of AI in chronic opioid users (mean duration of 60 months) leading them to suggest that adrenal function should be monitored in patients taking > 20 mg of morphine equivalents per day [113]. Benzodiazepines such as midazolam, oxazepam, alprazolam, and diazepam can also affect the HPA axis at the hypothalamic level [114–116]. Other drugs such as megestrol acetate and medroxyprogesterone acetate have GC activity with potential for HPA axis suppression [92]. In summary, careful review of the preoperative medications aimed at identifying drugs causing HPA axis impairment is critical. Recently, in the United Kingdom, a National Patient Safety Alert was issued stressing the importance of patients on chronic GCs to carry an emergency card to raise the awareness of the surgical team for appropriate perioperative GC management [117].
吗啡、羟考酮、芬太尼、曲马多、美沙酮和海洛因等阿片类药物被越来越多地认为是GC诱导AI的原因[112]。Li等人报道了慢性阿片类药物使用者(平均持续时间为60个月)中约9%的AI患病率,因此他们建议每天服用> 20mg吗啡当量的患者应监测肾上腺功能[113]。苯二氮卓类药物如咪达唑仑、恶西泮、阿普唑仑和地西泮也能影响下丘脑水平的下丘脑轴[114-116]。其他药物如醋酸甲孕酮和醋酸甲孕酮具有GC活性,可能抑制HPA轴[92]。总之,仔细检查术前用药,以确定引起下丘脑轴损伤的药物是至关重要的。最近,英国发布了全国患者安全警报,强调慢性GC患者携带急诊卡的重要性,以提高手术团队对GC围手术期适当管理的认识[117]。
Evaluation of Adrenal Function in Patients Using Glucocorticoids
Patients should undergo HPA testing if there is a plan to stop GC before surgery (see therapy section). Morning and random (during stress) serum cortisol levels and the CST are the most commonly used tests for the evaluation of the HPA axis. Despite some studies pointing toward suboptimal sensitivity of the CST (high false negative), and even though the idea of assessing the entire HPA axis (using insulin tolerance or Metyrapone test) vs the adrenal response to exogenous ACTH analog is attractive, the authors are not aware of any reports of patients who passed the CST and suffered cardiovascular collapse perioperatively [118–122]. The authors have taken care of a large number of patients with pituitary disorders who underwent surgery without GC coverage based on passing the CST. However, the CST may be unreliable in the first few weeks following an acute hypothalamic or pituitary event where the adrenal gland has not undergone enough atrophy [122–124].
如果术前计划停止GC,患者应接受HPA检测(见治疗部分)。早晨和随机(压力期间)血清皮质醇水平和CST是评估下丘脑轴最常用的测试。尽管一些研究指出CST的敏感性不佳(高假阴性),尽管评估整个HPA轴(使用胰岛素耐量或Metyrapone试验)与肾上腺对外源性ACTH类似物的反应的想法很有吸引力,但作者尚未发现任何通过CST并在围手术期发生心血管衰竭的患者的报道[118-122]。作者照顾了大量的垂体疾病患者,他们接受了手术,但没有基于通过CST的GC覆盖。然而,在急性下丘脑或垂体事件后的最初几周内,如果肾上腺没有经历足够的萎缩,CST可能不可靠[122-124]。
The laboratory evaluation of AI usually starts with obtaining a morning (7 am-9 am) serum cortisol measurement. Cortisol <3 µg/dL is diagnostic of AI; measuring ACTH levels will distinguish between primary and GC-induced AI [125]. If morning cortisol levels are in the indeterminate range (3-10 µg/dL), a CST may be performed [126]. A stimulated cortisol value of >14 to 15 µg/dL at 30 minutes after 250 ug of IV or intramuscular Cosyntropin excludes AI [127–129]. These cutoff values are suggested based on newer cortisol assays that use monoclonal antibodies and liquid chromatography with tandem mass spectrometry that result in about 20% to 0% lower cortisol levels compared to >18 µg/dL using polyclonal immunoassays [127–130]. Dehydroepiandrosterone sulfate (DHEA-S) may be used to further assess the HPA axis in patients with indeterminate serum cortisol levels [131]. A minimum DHEA-S level of 54 µg/dL rather than the lower limit of the normal range for the age has been suggested. One caveat is that any GC exposure in the past may result in long-term low DHEAS levels despite normal cortisol reserve.
AI的实验室评估通常从获得早晨(上午7点至9点)血清皮质醇测量开始。皮质醇<3µg/dL为AI诊断;测量ACTH水平可以区分原发性和GC诱导的AI[125]。如果早晨皮质醇水平在不确定的范围内(3-10µg/dL),则可以进行CST[126]。250 ug静脉注射或肌注Cosyntropin后30分钟刺激皮质醇值>14至15µg/dL排除AI[127-129]。这些截止值是基于使用单克隆抗体和液相色谱串联质谱的较新的皮质醇测定方法提出的,与使用多克隆免疫测定法>18 μ g/dL相比,该方法可使皮质醇水平降低约20%至0%[127-130]。硫酸脱氢表雄酮(DHEA-S)可用于进一步评估血清皮质醇水平不确定的患者的HPA轴[131]。建议最低DHEA-S水平为54微克/分升,而不是年龄正常范围的下限。一个警告是,任何GC暴露在过去可能导致长期低DHEAS水平,尽管正常的皮质醇储备。
Perioperative Glucocorticoid Management
Studies Evaluating the Effects of Supplemental Perioperative Glucocorticoids
Studies over the past several decades addressing the use of supplemental perioperative GCs in those on chronic GC therapy have major limitations. This has resulted in significant challenges to standardize clinical practice. As observed by Lamore et al, low-risk patients within the same institution often received hydrocortisone ranging between 50 and >200 mg/day for similar surgical risk, demonstrating a lack of agreement among clinicians [132].
在过去的几十年里,关于慢性GC患者围手术期使用辅助GCs的研究有很大的局限性。这给标准化临床实践带来了重大挑战。根据Lamore等人的观察,同一机构内的低风险患者通常接受50至>200毫克/天的氢化可的松治疗,手术风险相似,这表明临床医生之间缺乏共识[132]。
The question of how much cortisol is needed to prevent adrenal crisis is still unanswered. Based on the 3 randomized controlled trials (RCTs) [133–135], at least 6 cohort [118, 136–140], and 1 retrospective studies [141], in most cases continuing the daily preoperative GC regimen seems to be sufficient. The 3 RCTs had small sample sizes (between 17-92) and limitations, reflecting the need for additional studies in the field. Udelsman et al showed that in adrenalectomized primates who underwent open cholecystectomy, physiological GC replacement doses resulted in similar outcomes when compared to 10-fold supraphysiological doses [142]. In contrast, when a subphysiological dose (1/10 of the normal cortisol production rate) was given perioperatively, more hemodynamic instability with a higher mortality rate was observed [142]. In Table 4 we summarize the relevant clinical studies that support the notion that, in most cases, the current practice of perioperative GC administration is excessive and potentially harmful.
需要多少皮质醇来预防肾上腺危机的问题仍然没有答案。根据3项随机对照试验(RCTs)[133-135]、至少6项队列研究[118,136 - 140]和1项回顾性研究[141],在大多数情况下,继续每日术前GC方案似乎就足够了。这3项随机对照试验样本量较小(在17-92之间)且存在局限性,反映了该领域进一步研究的必要性。Udelsman等人的研究表明,在肾上腺切除的灵长类动物中,接受开放胆囊切除术,生理GC替代剂量与10倍的超生理剂量相比,结果相似[142]。相反,当围手术期给予亚生理剂量(正常皮质醇生成率的1/10)时,观察到更多的血流动力学不稳定和更高的死亡率[142]。在表4中,我们总结了相关的临床研究,这些研究支持这样一种观点,即在大多数情况下,目前围手术期给药的做法是过度的,并且可能有害。
Table 4. Studies addressing supplemental perioperative glucocorticoid treatment
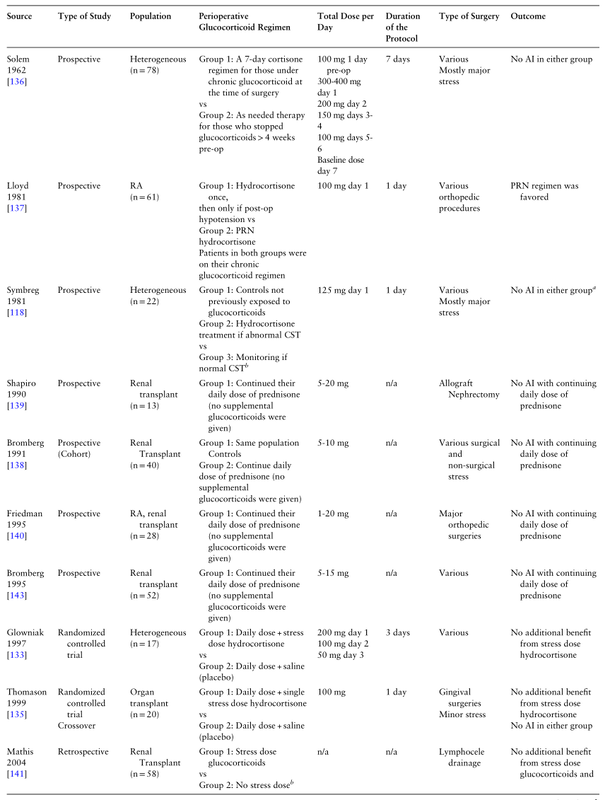
Table 4. Continued

Source Type of Study Population Perioperative
Glucocorticoid Regimen Total Dose per Day Duration of the Protocol Type of Surgery Outcome
Solem
1962 [136] Prospective Heterogeneous
(n = 78) Group 1: A 7-day cortisone regimen for those under chronic glucocorticoid at the time of surgery
vs
Group 2: As needed therapy for those who stopped glucocorticoids > 4 weeks pre-op 100 mg 1 day pre-op
300-400 mg day 1
200 mg day 2
150 mg days 3-4
100 mg days 5-6
Baseline dose day 7 7 days Various
Mostly major stress No AI in either group
Lloyd
1981 [137] Prospective RA
(n = 61) Group 1: Hydrocortisone once,
then only if post-op hypotension vs
Group 2: PRN hydrocortisone
Patients in both groups were on their chronic glucocorticoid regimen 100 mg day 1 1 day Various
orthopedic procedures PRN regimen was favored
Symbreg
1981 [118] Prospective Heterogeneous
(n = 22) Group 1: Controls not previously exposed to glucocorticoids
Group 2: Hydrocortisone treatment if abnormal CST
vs
Group 3: Monitoring if normal CSTb 125 mg day 1 1 day Various
Mostly major stress No AI in either groupa
Shapiro
1990 [139] Prospective Renal transplant
(n = 13) Group 1: Continued their daily dose of prednisone (no supplemental glucocorticoids were given) 5-20 mg n/a Allograft Nephrectomy No AI with continuing daily dose of prednisone
Bromberg
1991 [138] Prospective
(Cohort) Renal Transplant
(n = 40) Group 1: Same population Controls
Group 2: Continue daily dose of prednisone (no supplemental glucocorticoids were given) 5-10 mg n/a Various surgical and non-surgical stress No AI with continuing daily dose of prednisone
Friedman
1995 [140] Prospective RA, renal transplant
(n = 28) Group 1: Continued their daily dose of prednisone (no supplemental glucocorticoids were given) 1-20 mg n/a Major orthopedic surgeries No AI with continuing daily dose of prednisone
Bromberg
1995 [143] Prospective Renal transplant
(n = 52) Group 1: Continued their daily dose of prednisone (no supplemental glucocorticoids were given) 5-15 mg n/a Various No AI with continuing daily dose of prednisone
Glowniak
1997 [133] Randomized controlled trial Heterogeneous
(n = 17) Group 1: Daily dose + stress dose hydrocortisone
vs
Group 2: Daily dose + saline (placebo) 200 mg day 1
100 mg day 2
50 mg day 3 3 days Various No additional benefit from stress dose hydrocortisone
Thomason
1999 [135] Randomized controlled trial
Crossover Organ
transplant
(n = 20) Group 1: Daily dose + single stress dose hydrocortisone
vs
Group 2: Daily dose + saline
(placebo) 100 mg 1 day Gingival surgeries
Minor stress No additional benefit from stress dose hydrocortisone
No AI in either group
Mathis
2004 [141] Retrospective Renal Transplant
(n = 58) Group 1: Stress dose glucocorticoids
vs
Group 2: No stress doseb n/a n/a Lymphocele drainage No additional benefit from stress dose glucocorticoids and more hyperglycemia was reported
Zaghiyan
2011 [144] Retrospective IBD
(n = 49) Group 1: Stress dose glucocorticoids
vs
Group 2: No stress dose 100 mg (incision)
300 mg day 1
Taper to prednisone 20 mg per day 4 days Major abdominal surgeries No additional benefit from stress dose glucocorticoids
No AI in either group
Zaghiyan
2012 [145] Prospective IBD
(n = 32) Group 1: Patient not currently on glucocorticoid (but previously exposed during the past 12 months) received no treatment perioperatively
vs
Group 2: Patients currently on glucocorticoids receiving low-dose supplemental glucocorticoids perioperatively One-third of pre-op dose (incision)
One-third of the dose Q8hrs day of surgery
One-fourth Q8hr day 1
One-sixth Q8hr day 2
One-fourth Q12hr day 3
Prednisone equivalent day 4 4 days Major abdominal surgeries No AI in either group
Low-dose supplemental glucocorticoid regimens seem safe
Zaghiyan
2012 [146] Retrospective IBD
(n = 97) Group 1: High (stress) dose glucocorticoids
vs
Group 2: Low-dose supplemental glucocorticoid (see [145]) 100 mg (Incision)
300 mg day 1
Taper to prednisone 4 days Major abdominal surgeries No AI in either group
No additional benefit from high-dose stress glucocorticoids
Ayatac
2013 [147] Retrospective IBD
(n = 235) Group 1: Daily dose + Stress dose glucocorticoids
vs
Group 2: Daily dose (unless off glucocorticoids preoperatively) + no stress dose 100 mg (operating room)
300 mg day 1
Then taper n/a Major abdominal surgeries One case of AI in group 1.
No additional benefit or harm was seen from stress dose glucocorticoids
Zaghiyan
2014 [134] Single blinded randomized controlled trial IBD
(n = 92) Group 1: Stress dose hydrocortisone
vs
Group 2: Hydrocortisone equivalent to oral daily dose 100 mg (incision)
300 mg day 1
225 mg day 2
150 mg day 3
100 mg day 4
20 mg Prednisone 5 days Major abdominal surgeries No additional benefit from stress dose glucocorticoids
Excluded studies where both chronic glucocorticoids and supplemental treatment were not provided [33, 148].
AI, adrenal insufficiency; CST, cosyntropin stimulation test; IBD, inflammatory bowel disease; n/a, not available; RA, rheumatoid arthritis.
a 125 mg resulted in higher cortisol levels than nonexposed.
b Unclear if patients were receiving or stopped chronic glucocorticoids at the time of surgery.
The first RCT studied 17 patients on prednisone 5 to 60 mg/day for a duration of 2 months to 20 years [133]. All patients had abnormal CST. These patients were kept on their daily dose of prednisone and randomized to saline injections vs stress doses of 200 mg of hydrocortisone with a gradual taper over 3 days. No adrenal crisis was observed in the group on the daily dose of GC and placebo [133]. In the second RCT, 20 solid organ transplant patients receiving maintenance doses of prednisone (5-10 mg/day) were randomized to continue their chronic GC regimen vs additional 100 mg of hydrocortisone during gingival surgery under local anesthesia. No significant difference in hypotension or signs of AI was observed in either group [135]. Lastly, a single blinded RCT included 92 patients with IBD undergoing major colorectal surgery who were randomized to receive either hydrocortisone 100 mg every 8 hours. followed by a taper, or IV hydrocortisone equivalent dose of the presurgical GC regimen [134]. As with the previous RCTs, there was no evidence of hemodynamic benefit from supplemental stress dose GCs.
第一项随机对照试验研究了17例泼尼松5 - 60mg /天,持续2个月至20年的患者[133]。所有患者CST均异常。这些患者保持每日剂量的强的松,并随机分为生理盐水注射和200mg氢化可的松应激剂量,并在3天内逐渐减少剂量。每日剂量GC和安慰剂组未观察到肾上腺危机[133]。在第二项随机对照试验中,20名接受维持剂量强的松(5-10 mg/天)的实体器官移植患者在局部麻醉下进行牙龈手术,随机分为两组,一组继续慢性GC方案,另一组额外100 mg氢化可的松。两组患者的低血压和AI症状均无显著差异[135]。最后,一项单盲随机对照试验纳入了92名接受大肠癌手术的IBD患者,他们被随机分配每8小时接受100毫克氢化可的松治疗。随后逐渐减少或静脉注射等量氢化可的松手术前GC方案[134]。与之前的随机对照试验一样,没有证据表明补充应激剂量的GCs对血流动力学有好处。
The retrospective and nonrandomized prospective studies support the RCTs’ results. In kidney transplant patients on an average prednisone dose of 15.5 mg/day for >84 days, adding stress dose GCs to their chronic GC regimen did not provide additional hemodynamic benefit during minor surgical procedures but increased the risk of hyperglycemia [141]. In another study, 40 renal transplant patients on GCs for at least 3 months were admitted to the hospital for various reasons (sepsis, metabolic abnormalities, surgery). They continued their daily prednisone regimen of 5 to 10 mg/day without supplemental treatment. When comparing this group to the same population of patients with renal allograft admitted to the hospital for various reasons during the same period of time, there was no increased mortality, length of hospital stay, or adrenal crises [138]. Other groups consistently demonstrated a lack of benefit of stress dose GCs in patients with IBD undergoing major abdominal surgery [76, 144–147].
回顾性和非随机前瞻性研究支持随机对照试验的结果。在平均泼尼松剂量为15.5 mg/天、持续>84天的肾移植患者中,在慢性GC方案中加入应激剂量的GC并没有在小手术过程中提供额外的血流动力学益处,反而增加了高血糖的风险[141]。在另一项研究中,40例接受GCs治疗至少3个月的肾移植患者因各种原因(败血症、代谢异常、手术)入院。他们继续每日5 - 10mg /天的强的松治疗,没有补充治疗。将该组与同一时期因各种原因住院的同种异体肾移植患者进行比较,死亡率、住院时间或肾上腺危机均未增加[138]。其他组一致表明,应激剂量GCs对接受腹部大手术的IBD患者缺乏益处[76,144 - 147]。
In general, the literature supports the concept that continuing the daily GC dose in patients suspected to have GC-induced AI is sufficient to prevent adrenal crises perioperatively. In fact, in the only 3 confirmed postoperative adrenal crisis-related deaths, chronic supraphysiological GC doses were stopped before surgery [8]. Based on the level of surgical stress, parenteral GC treatment in patients who cannot tolerate oral intake is reasonable with a gradual return to daily GC dose once daily intake is resumed (Table 5).
总的来说,文献支持这样一种观点,即在怀疑有GC诱导的AI的患者中继续每日GC剂量足以预防围手术期肾上腺危像。事实上,在仅有的3例确诊的术后肾上腺危象相关死亡中,慢性supraphysiological的GC剂量在术前停止使用[8]。根据手术应激水平,对于不能耐受口服的患者,肠外GC治疗是合理的,一旦恢复每日摄入,逐渐恢复每日GC剂量(表5)。
Table 5. Perioperative treatment regimens suggested for patient with glucocorticoid-induced AI

Regimen Degree of Surgical Stress Glucocorticoid Regimen
Patients currently on glucocorticoids
Garde I Minor
Continue daily dose of glucocorticoid
25 mg of IV hydrocortisone at induction if not able to tolerate PO
Resume oral daily preoperative glucocorticoid regimen
Grade II Moderate
Continue daily dose of glucocorticoid
25-50 mg of hydrocortisone IV at induction
15-25 mg hydrocortisone every 6 hours. until PO is tolerated and hemodynamically stable
Resume oral daily preoperative glucocorticoid regimen
Grade III Major
Continue daily dose of glucocorticoid
50 mg of hydrocortisone IV at induction
25 mg of hydrocortisone IV every 6 hours on day 1 and until hemodynamically stable, then 15 mg IV every 6 hours until PO is tolerateda
Resume oral daily preoperative glucocorticoid regimen
Patients who stopped or plan to stop glucocorticoids before surgery
Assess HPA axis in patients with intermediate to high risk (see Table 4)
The closer the date of discontinuing glucocorticoids before surgery, the higher the risk of AI
Treat based on the degree of surgical stress in those who have abnormal HPA axis
Adrenal crisis
100 mg of hydrocortisone IV (IM if no IV access)
50 mg every 6 hours until hemodynamically stable and then tapera
Taper depending on clinical response-intravenous fluids (normal saline), dextrose 5% if hypoglycemia
These recommendations include the authors’ personalized approach to perioperative management in patients with glucocorticoid-induced AI.
AI, adrenal insufficiency; HPA, hypothalamic-pituitary-adrenal; IM, intramuscular; IV, intravenous; PO, by mouth.
Some experts favor continuous glucocorticoid infusion.
Glucocorticoid “Coverage”
There have been a large number of supplemental perioperative GC regimens recommended over the past decades [6, 8, 11, 17, 66–156]. Some of these recommendations were based on estimated cortisol production rates during stress and others empirically based. Simultaneously, experts in the field have consistently voiced their concerns about the excess amount of GC coverage, which may be harmful (Table 6). Awareness of the lack of evidence to support the use of high-dose perioperative GCs and the associated risks has resulted in a trend to recommend lower doses and shorter duration of these protocols [12–14]—from >300 mg of hydrocortisone [118] to about 100 to 200 mg/day [11, 66, 70, 150, 156] and shorter tapers over <7 days—without increased mortality.
在过去的几十年里,有大量的围手术期补充GC方案被推荐[6,8,11,17,66 - 156]。其中一些建议是基于stress时估计的皮质醇生成率,而另一些则是基于经验。同时,该领域的专家一直对GC覆盖过量表示担忧,这可能是有害的(表6)。由于缺乏证据支持使用高剂量围手术期GC及其相关风险,因此有一种趋势,建议使用较低剂量和较短持续时间[12-14]——从>300毫克氢化可的松[118]到约100至200毫克/天[11,66,70,150,156]。和小于7天的较短的tapers over没有增加死亡率。
Table 6. Review articles that commented on perioperative glucocorticoid treatment regimen

Source Year Comments from the Authors Conclusions on Treatment
Kehlet [151] 1975 “Glucocorticoid should only be given in a necessary and adequate dose” to avoid side effects Most regimens are founded on empirical basis
Salem [8] 1994 The risk should be individualized based on the glucocorticoid preoperative dose, duration, and type of surgery We are giving too much glucocorticoids
De Lange [157] 2008 There is no evidence to support excessive dosing (>200 mg hydrocortisone equivalent/day) or extensive duration in uncomplicated cases We are giving too much glucocorticoids
Marik [158] 2008 “Stress doses are not routinely required as long as the patient continues their usual daily dose of glucocorticoids” We are giving too much glucocorticoids
Fleager [155] 2010 “There are no evidence-based treatment guidelines that provide firm recommendations for the administration of perioperative steroids” No evidence for current practice
Kelly [159] 2013 Based on the existing evidence, patients on long-term glucocorticoids do not require the once-standard high doses; just continue their maintenance doses perioperatively. Treat refractory hypotension with rescue doses of steroids We are giving too much glucocorticoids
Hicks [160] 2015 “Current prescribing practices are highly variable, likely because of a lack of randomized controlled data and a wide range of preoperative treatment regimens” “Recent data suggest that additional corticosteroid supplementation in the perioperative period may be unnecessary and may serve only to increase the risk of poor wound healing and infectious”
MacKenzie [161] 2016 “Despite little evidence for this practice (supraphysiological supplemental perioperative glucocorticoids), few have challenged this treatment paradigm” “With few exceptions, the use of supraphysiologic glucocorticoid therapy for adults with presumed adrenal insufficiency due to exogenous glucocorticoid use should be regarded as unnecessary”
Liu [156] 2017 “Recommendations in major textbooks are confusing, inconsistent, and lacking in class A or B evidence” There is no universal agreement regarding dose, duration, or regimen of supplemental glucocorticoid
Groleau [9] 2018 “It is not possible to conclude that perioperative administration of corticosteroids, compared to placebo, reduced the incidence of adrenal insufficiency” Providing the daily maintenance dose without supplemental glucocorticoids may be sufficient
Khazen [162] 2018 “We found no evidence to support the use of supraphysiologic dose of glucocorticoid therapy provided the patient receive their usual dose of glucocorticoid preoperatively” “A well-designed, large multicenter RCT is warranted”
Chilkoti [66] 2019 “There are no dogmatic guidelines regarding perioperative “stress dose” of steroids in patients on chronic steroid therapy; however, there is enough evidence that patients on long-term exogenous steroid therapy do not require the conventional high-dose perioperative corticosteroid, instead must be kept on their baseline maintenance dose” We are giving too much glucocorticoids
Manou-Stathopoulou [163] 2019 “Clinical trials exploring glucocorticoid supplementation have provided conflicting data, reflecting the lack of understanding of the cortisol biology during the perioperative period” More personalized targeted therapies are needed
Seo [11] 2021 Many clinical trials have low level of evidence, lack of power, without clear criteria for AI that results in high variation in the recommendations No evidence for current practice
Laugesen [10] 2021 “Current evidence indicates substantial variation regarding risk and course of glucocorticoid-induced adrenal insufficiency … more research is needed to refine the diagnosis and to support evidence-based clinical decision-making” No evidence for current practice
Many review articles discuss the topic and make recommendations without specific comments about the current practice.
AI, adrenal insufficiency; RCT, randomized controlled trial.
In a study by Arafah [164], 20 mg of oral hydrocortisone 2 to 4 hours. prior to surgery resulted in a baseline cortisol of 14.8 ug/dL in those with central AI. The administration of 25 mg of hydrocortisone IV resulted in a nadir serum cortisol range of 16 to 34 ug/dL at 6 hours, which was higher with subsequent injections. In this setting, cortisol half-life was longer, volume of distribution increased, and clearance was lower in patients with AI compared to healthy individuals. This study suggests that patients with AI who are administered 20 mg of hydrocortisone 2 to 4 hours prior to intubation have a baseline cortisol level comparable to healthy individuals with an intact HPA axis. Subsequently, providing 25 mg of IV hydrocortisone every 6 hours for 24 hours, followed by 15 mg every 6 hours or 24 hours, resulted in no adverse events or symptoms suggestive of AI. This study did not include patients with GC-induced AI, but the same concepts may be applied as most subjects in this study had secondary AI. Additionally, cardiac surgery procedures were not included. The results are in agreement with the trend that our current practices provide higher perioperative GC doses than needed [165]. Considering this, 15 to 25 mg of hydrocortisone IV every 6 hours (60-100 mg/day) should provide enough perioperative coverage for even moderate to major operations in patients suspected to have GC-induced AI. Others have shown that continuous hydrocortisone infusion provides more stable cortisol concentrations during major stress without significant peaks and troughs that may be seen with intramuscular or IV administrations. This approach requires an additional IV infusion line, and no data indicate that continuous hydrocortisone infusion prevents adrenal crisis or is associated with lower adverse events compared to intermittent hydrocortisone injections [70]. We have summarized our perioperative approach in Table 5.
在Arafah[164]的一项研究中,20毫克氢化可的松口服2至4小时。手术前导致中枢性AI患者的基线皮质醇为14.8 ug/dL。给药25mg氢化可的松IV导致血清皮质醇在6小时的最低点范围为16至34 ug/dL,随后的注射更高。在这种情况下,与健康个体相比,AI患者的皮质醇半衰期更长,分布体积增加,清除率更低。该研究表明,AI患者在插管前2至4小时给予20mg氢化可的松,其基线皮质醇水平与HPA轴完整的健康个体相当。随后,每6小时给予25mg静脉注射氢化可的松,持续24小时,随后每6小时或24小时给予15mg静脉注射氢化可的松,没有导致不良事件或提示AI的症状。本研究没有纳入GC诱导的AI患者,但由于本研究中大多数受试者都有继发性AI,因此可以应用相同的概念。此外,心脏手术也不包括在内。结果与我们目前的做法提供的围手术期GC剂量高于所需的趋势一致[165]。考虑到这一点,每6小时15 - 25mg氢化可的松IV (60- 100mg /天)应提供足够的围手术期覆盖,即使是中等到大的手术,怀疑有GC诱导的AI患者。其他研究表明,持续的氢化可的松输注在主要压力期间提供更稳定的皮质醇浓度,没有明显的峰值和低谷,可能会在肌肉注射或静脉注射中看到。这种方法需要额外的静脉输液线,并且没有数据表明持续的氢化可的松输注可以预防肾上腺危机,或者与间歇性的氢化可的松注射相比,不良事件发生率更低[70]。我们在表5中总结了围手术期方法。
There is little data in perioperative management of pregnant patients on chronic GC. Cortisol levels increase throughout pregnancy secondary to increased CBG and to the HPA axis stimulation by placental CRH. The dose of GC replacement does not usually need to be increased during the first and second trimesters, but an increase in GC dosage of 20% to 40% from the 24th week forward is generally recommended [166]. Accordingly, a 50% higher perioperative parenteral GC coverage, especially during the third trimester, and delivery seems reasonable. Careful perioperative monitoring of the hemodynamic status of pregnant women and their fetus is critical [166–168].
妊娠期慢性GC患者的围手术期处理资料较少。皮质醇水平在整个妊娠期间增加,继发于CBG增加和胎盘CRH刺激HPA轴。在妊娠早期和中期,通常不需要增加GC替代剂量,但一般建议从第24周开始增加20%至40%的GC剂量[166]。因此,围手术期肠外GC覆盖率提高50%,特别是在妊娠晚期,分娩似乎是合理的。围手术期仔细监测孕妇及其胎儿的血流动力学状态至关重要[166-168]。
Conclusions
Perioperative management of patients on GCs has been a major topic of discussion for over 70 years. It is clear that assessment of the HPA axis in patients who have stopped GC therapy before surgery is necessary. However, despite the progress in our understanding of the stress response and hormonal behavior perioperatively, there is significant heterogeneity in clinical practice in terms of GCs dosing. In most cases, excess GCs are administered, which may result in several adverse events. The current literature supports that in patients undergoing a surgical procedure, continuing the daily dose of GCs along with a short course of perioperative IV GCs based on the level of anticipated surgical stress is adequate. In most perioperative scenarios, administration of ≤100 mg/day hydrocortisone with a rapid taper to preoperative GC dose is sufficient. Close monitoring for any evidence of hemodynamic instability is fundamental. Finally, there is a need for large prospective studies to optimize the perioperative management of patients on GCs to avoid any clinically significant AI-related event and do no harm.
70多年来,GCs患者的围手术期管理一直是一个重要的讨论话题。很明显,在手术前停止GC治疗的患者中评估下丘脑轴是必要的。然而,尽管我们对围手术期应激反应和激素行为的理解有所进展,但在临床实践中,在GCs的剂量方面存在显著的异质性。在大多数情况下,使用过量的GCs,这可能导致一些不良事件。目前的文献支持,在接受外科手术的患者中,根据预期的手术应激水平,继续每日剂量的GCs以及围手术期短疗程的静脉GCs是足够的。在大多数围手术期情况下,≤100mg /天的氢化可的松,并迅速减少到术前GC剂量就足够了。密切监测任何血流动力学不稳定的证据是至关重要的。最后,需要大量的前瞻性研究来优化GCs患者的围手术期管理,以避免任何临床显著的AI相关事件,并且不造成伤害。
Abbreviations
AI adrenal insufficiency
CST cosyntropin stimulation test
DHEA-S dehydroepiandrosterone sulfate
GC glucocorticoid
HPA hypothalamic-pituitary-adrenal
IBD inflammatory bowel disease
RCT randomized controlled trial

本文为转载文章,如有侵权请联系作者删除。本文仅供健康科普使用,不能做为诊断、治疗的依据,请谨慎参阅





评论