
髋关节撞击成像的里斯本Lisbon共识-第三部分:成像技术(2021)
髋关节撞击成像的里斯本Lisbon共识-第三部分:成像技术(2021)The Lisbon Agreement on Femoroacetabular Impingement Imaging-part 3: imaging techniques
Castro M O, Mascarenhas V V, Afonso P D, Rego P, Schmaranzer F, Sutter R, Kassarjian A, Sconfienza L, Dienst M, Ayeni O R, Beaule P E, Dantas P, Lalam R, Weber M A, Vanhoenacker F M, Dietrich T J, Jans L, Robinson P, Karantanas A H, Sudol-Szopinska I, Anderson S, Noebauer-Huhmann I, Marin-Pena O, Collado D, Tey-Pons M, Schmaranzer E, Padron M, Kramer J, Zingg P O, De Maeseneer M, Llopis E. The Lisbon Agreement on Femoroacetabular Impingement Imaging-part 3: imaging techniques[J]. Eur Radiol, 2021,31(7): 4652-4668.
转载文章的原链接1:
https://pubmed.ncbi.nlm.nih.gov/33411053/
转载文章的原链接2:
https://air.unimi.it/handle/2434/804172
Abstract
Objectives
Imaging diagnosis of femoroacetabular impingement (FAI) remains controversial due to a lack of high-level evidence, leading to significant variability in patient management. Optimizing protocols and technical details is essential in FAI imaging, although challenging in clinical practice. The purpose of this agreement is to establish expert-based statements on FAI imaging, using formal consensus techniques driven by relevant literature review. Recommendations on the selection and use of imaging techniques for FAI assessment, as well as guidance on relevant radiographic and MRI classifications, are provided.
由于缺乏高水平的证据,髋关节撞击(FAI)的影像学诊断仍然存在争议,导致患者管理的显著差异。优化方案和技术细节对FAI成像至关重要,尽管在临床实践中具有挑战性。本协议的目的是在相关文献综述的推动下,使用正式的共识技术,建立基于专家的FAI成像声明。提供了关于FAI评估的成像技术的选择和使用的建议,以及有关放射学和MRI分类的指导。
Methods
The Delphi method was used to assess agreement and derive consensus among 30 panel members (musculoskeletal radiologists and orthopedic surgeons). Forty-four questions were agreed on and classified into five major topics and recent relevant literature was circulated, in order to produce answering statements. The level of evidence was assessed for all statements and panel members scored their level of agreement with each statement during 4 Delphi rounds. Either “group consensus,” “group agreement,” or “no agreement” was achieved.
采用德尔菲法评估30名小组成员(肌肉骨骼放射科医生和骨科医生)的一致性并得出共识。商定了44个问题,并将其分为五个主要专题,分发了最近的有关文献,以便作出答复。对所有陈述的证据水平进行评估,并在4轮德尔菲中对小组成员对每个陈述的同意程度进行评分。达成了“小组共识”、“小组协议”或“没有协议”。
Results
Forty-seven statements were generated and group consensus was reached for 45. Twenty-two statements pertaining to “Imaging techniques” were generated. Eight statements on “Radiographic assessment” and 12 statements on “MRI evaluation” gained consensus. No agreement was reached for the 2 “Ultrasound” related statements.
产生了47项声明,达成了45项小组共识。产生了22份关于“成像技术”的陈述。关于“X线评估”的8项声明和“MRI评估”的12项声明达成共识。两份与“超声波”相关的声明没有达成一致。
Conclusion
The first international consensus on FAI imaging was developed. Researchers and clinicians working with FAI and hip-related pain may use these recommendations to guide, develop, and implement comprehensive, evidence-based imaging protocols and classifications.
在FAI成像方面形成了第一个国际共识。研究FAI和髋关节相关疼痛的研究人员和临床医生可以使用这些建议来指导、开发和实施全面的、基于证据的成像方案和分类。
Key Points
• Radiographic evaluation is recommended for the initial assessment of FAI, while MRI with a dedicated protocol is the gold standard imaging technique for the comprehensive evaluation of this condition.
• The MRI protocol for FAI evaluation should include unilateral small FOV with radial imaging, femoral torsion assessment, and a fluid sensitive sequence covering the whole pelvis.
• The definite role of other imaging methods in FAI, such as ultrasound or CT, is still not well defined.
•对于FAI的初步评估建议采用影像学评估,而具有专用方案的MRI是全面评估该病症的金标准成像技术。
•FAI评估的MRI方案应包括单侧小视场中心区域的成像、股骨扭转评估和覆盖整个骨盆的流体敏感序列。
•其他成像方法(如超声或CT)在FAI中的确切作用尚不明确。
Introduction
Femoroacetabular impingement (FAI) is a motion-related clinical disorder associated with the insidious onset of groin/hip pain, along with signs of limited motion and characteristic imaging findings [1, 2], as a result of abnormal contact between the proximal femur and acetabular rim [1]. This conflicting motion may ultimately lead to premature osteoarthritis (OA) of the hip [3, 4].
While the presence of characteristic femoral (Cam) and/or acetabular (Pincer) morphologies on imaging is necessary to diagnose FAI [2], along with clinical findings, several imaging aspects of FAI remain largely not standardized due to a lack of high-level evidence and little agreement amongst the radiology and orthopedic communities on which imaging modalities and parameters should be routinely used [5, 6], leading to significant variability among practitioners and institutions. Furthermore, chondrolabral damage is heterogeneously assessed using unenhanced MRI, MR arthrography (MRA), and CT arthrography (CTA) [7,8,9], while different systems of classification of such damage exist [10, 11]. Additionally, given that signs of OA may preclude surgical treatment of FAI, several imaging modalities and classifications are used in an attempt to define the best surgical candidates, albeit with variable reliability [12,13,14].
Accordingly, there is a need for improvement and standardization of diagnostic and treatment algorithms in FAI. Collecting expert views in a structured and systematic manner is a valid method of identifying current medical opinions in areas where scientific evidence is conflicting or unavailable. The aim of this Delphi-based consensus, “The Lisbon Agreement on FAI Imaging”, is thus to establish expert-based statements on imaging of FAI, by using formal techniques of consensus building among a group of specialists, driven by the results of relevant literature review. Protocols and technical parameters are the focus of this paper, which presents expert-based assertions on the selection and use of imaging techniques for FAI evaluation, as well as recommendations on relevant MRI and radiographic classifications. Other topics of the consensus initiative were discussed elsewhere.
Methods
This paper is part of a multidisciplinary project aiming to establish expert-based consensus on FAI imaging, entitled “The Lisbon Agreement on Femoroacetabular Impingement Imaging.” Briefly, after project conception (by V.V.M., M.O.C. and P.D.A.), the Delphi method was used to formally assess consensus in 4 rounds involving 30 radiology and orthopedic surgery experts.
Forty-four questions were agreed on and classified into five major topics and recent relevant literature was circulated, in order to produce answering statements. A total of 47 statements were generated and distributed among the topics “General issues” (9 statements), “Parameters and reporting” (16 statements), “Radiographic assessment” (8 statements), “MRI evaluation” (12 statements), and “Ultrasound” (2 statements). The level of evidence was assessed for all statements. Panel members scored their level of agreement (0 to 10) with each statement. Minimum compliance level was considered by the panel if a median ≥ 8 and interquartile range < 4 were achieved. For these statements, “Group consensus” was further defined as ≥ 80% of voters scoring ≥ 8, and “Group agreement” defined as 67–79% of voters scoring ≥ 8. “Lack of agreement” was assigned if the previous conditions were not met.
Panel members gathered in four meetings that sought to give feedback on the project status, discuss ideas, and keep group members engaged. Finally, panel members gave presentations on several consensus items during the European Society of Skeletal Radiology (ESSR) 2019 annual meeting, held in Lisbon, Portugal.
Full details on these aspects, including participants, consensus technique, literature review, statement drafting, levels of evidence, final scoring and data analysis are provided as Supplementary material.
Due to the importance and scope of collected information, consensus items were combined in a three-part series for publication. In the current work, we report results concerning part 3 (Imaging Techniques) and include the topics “Radiographic assessment,” “MRI evaluation,” and “Ultrasound,” followed by a summary of the panel’s discussion. The remaining items have recently been published in part 2 (“The Lisbon Agreement on Femoroacetabular Impingement Imaging—part 2: general Issues, parameters, and reporting,” Mascarenhas et al, European Radiology, DOI 10.1007/s00330-020-07432-1), while a selection of the most relevant statements was the subject of a first publication (“The Lisbon Agreement on Femoroacetabular Impingement Imaging—part 1: overview,” Mascarenhas et al, European Radiology, DOI 10.1007/s00330-020-06822-9).
Results and discussion
A total of 30 experts (21 musculoskeletal radiologists and 9 orthopedic surgeons) were included. Of these, 26 participants completed the 4 rounds of the survey.
At the end of the Delphi process, “group consensus” was obtained for 45 statements. Although level 2 evidence exists regarding the use of ultrasound in the evaluation of FAI, no agreement was reached for the 2 statements on this imaging technique, reflecting the non-established role of ultrasound in the diagnosis of FAI. Currently, its definite indication in FAI is to guide diagnostic and therapeutic hip joint injections [15]. More details on these aspects are provided as Supplementary material.
The pathway for the work-up of suspected FAI (Fig. 1), along with statements on imaging techniques and relevant classifications (Tables 1 and 2), was put forth by the panel.
Fig. 1 Overview of the work-up of a suspected FAI patient, focusing on the role of different imaging modalities. Radiographs are used in first-line assessment of FAI morphologies and signs of osteoarthritis (OA), while MRI provides a more comprehensive evaluation, including detailed bone morphology and evaluation of intra-articular soft tissue structures. Currently, the role of ultrasound is limited to the guidance of hip injections. AP, anteroposterior; w, with; wo, without
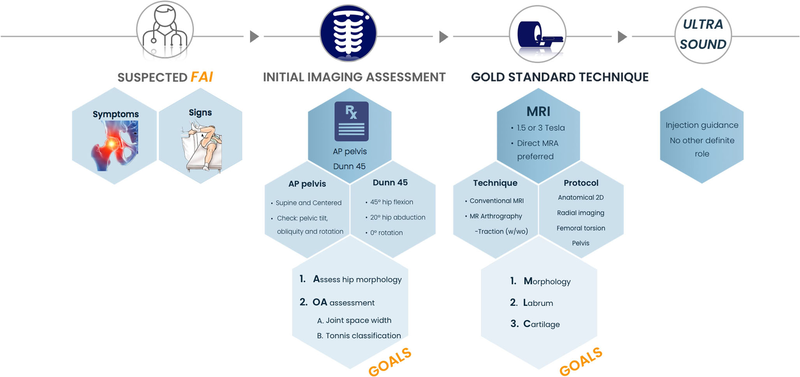
Table 1 Panel statements on radiographs and MRI in FAI with evidence levels. All presented statements obtained group consensus. The listed levels of agreement represent the percentage of votes ≥ 8 on a 0–10 scale. AP, anteroposterior; FAI, femoroacetabular impingement; FHN, femoral head-neck; FOV, field-of-view; IQR, interquartile range; MRI, magnetic resonance imaging; L-CEA, lateral center-edge angle; LOE, level of evidence; Q1, 1st quartile; Q3, 3rd quartile; T, Tesla
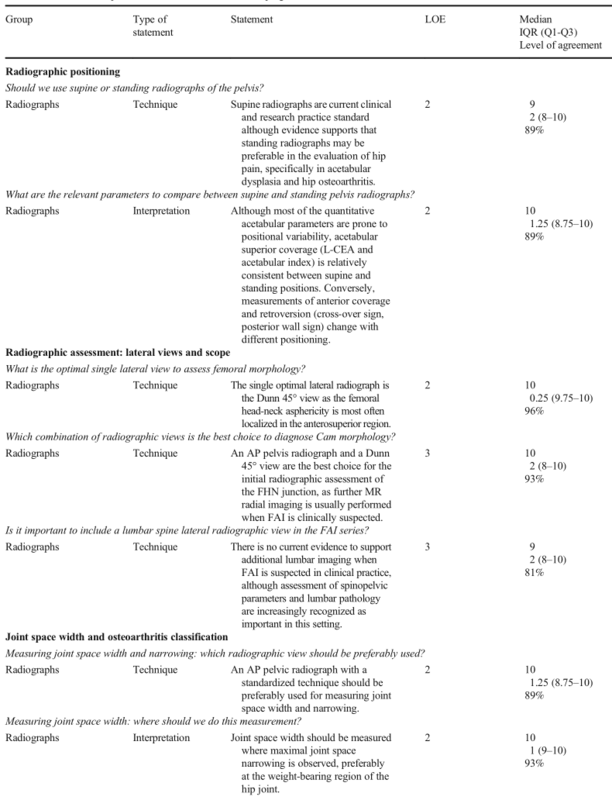
Table 1 (continued)

Table 1 (continued)
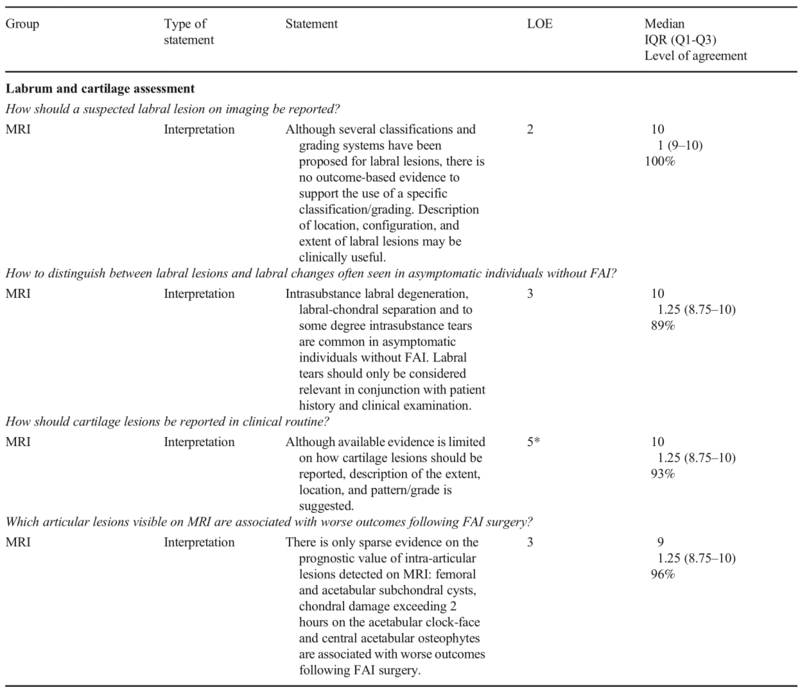
*Level of evidence 5 represents expert opinion
Table 2 Statements on the role of ultrasound in FAI, with evidence levels. The listed levels of agreement represent the percentage of votes ≥ 8 on a 0–10 scale. None of these statements obtained group agreement. FAI, femoroacetabular impingement; FHN, femoral head-neck; IQR, interquartile range; LOE, level of evidence; Q1, 1st quartile; Q3, 3rd quartile; US, ultrasound
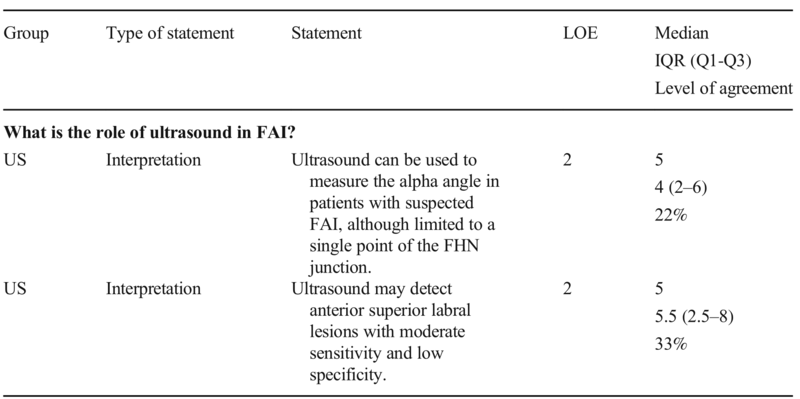
Radiographs
Radiographic positioning
Statement:
Supine radiographs are current clinical and research practice standard although evidence supports that standing radiographs may be preferable in the evaluation of hip pain, specifically in acetabular dysplasia and hip osteoarthritis.
Statement:
Although most of the quantitative acetabular parameters are prone to positional variability, acetabular superior coverage (L-CEA and acetabular index) is relatively consistent between supine and standing positions. Conversely, measurements of anterior coverage and retroversion (cross-over sign, posterior wall sign) change with different positioning.
From supine to weight-bearing radiographs, significant positional pelvic changes occur [16,17,18]. Standing radiographs are typically associated with increased pelvis extension (i.e., posterior pelvic tilt or sacral verticalization) [16,17,18,19] and decreased measures of anterior coverage [16,17,18, 20]. Although some imaging parameters are prone to change between supine and standing positions due to pelvic extension, from the clinical standpoint, acetabular measurements of superior coverage (the L-CEA, AI, extrusion index, and Sharp angle) are relatively consistent between projections [16, 21]. On the other hand, measurements of anterior coverage and retroversion (anterior acetabular coverage, posterior acetabular coverage, cross-over sign, posterior wall sign, and retroversion index) significantly change with pelvic tilt/rotation and consequently with different radiographic projections/positions [17, 19, 21] (Supplementary Table 1 and Supplementary Figure 1).
As such, in clinical entities in which acetabular evaluation is essential, such as Pincer FAI and acetabular dysplasia, weight-bearing AP pelvic radiographs, although technically more challenging, have been advocated since they reflect functional anatomical positioning [16, 18, 21]. The major drawback of this approach is related to the less straightforward comparison between standing preoperative radiographic assessment and fluoroscopy monitoring during surgery [22]. Additionally, most outcome studies and measurement thresholds are derived from supine AP radiographs. Surgeons and radiologists should be aware of these constraints [16, 21, 22] (Supplementary Table 2). Caution is warranted in borderline cases where small angular changes could be relevant to clinical decision-making. In such cases, a more comprehensive imaging evaluation should be undertaken.
In the assessment of hip OA, standing radiographs also seem to be preferable, although available data are not conclusive [23, 24].
Radiographic assessment
a. Lateral hip views and Cam morphology assessment
Statement:
The single optimal lateral radiograph is the Dunn 45° view as the femoral head-neck asphericity is most often localized in the anterosuperior region.
Statement:
An AP pelvis radiograph and a Dunn 45° view are the best choice for the initial radiographic assessment of the FHN junction, as further MR radial imaging is usually performed when FAI is clinically suspected.
Presently, radiographs and MRI are the standard imaging modalities used to characterize hip pathomorphology and to plan treatment [2, 25]. For an initial diagnostic approach, AP pelvis and lateral radiographs [2, 6] have been traditionally used (Supplementary Table 3).
Femoral head-neck (FHN) asphericity in hips with FAI is usually localized anterosuperiorly [26, 27]. Although not unanimously accepted, these asphericities are usually best shown radiographically in a Dunn 45° view (hips in 45° of flexion and 20° of abduction) [28,29,30], which is the recommended lateral view by the consensus panel (Supplementary Figure 2).
Different authors have proposed other combinations of radiographic projections. Some have demonstrated that the use of a three-view series (AP pelvis, Dunn 45°, and frog-leg lateral; Dunn 45°, Dunn 90°, and cross-table) [30] or a two-view series (Meyer lateral and Dunn 90°) [31] is ideal for the radiographic evaluation of Cam morphology. However, the alpha angle and head-neck offset measurements from these and other views were reported to describe no more than 50% of the overall variation in the shape of the proximal femur [31]. Given that the hip is a 3D structure, pre-arthritic hip conditions are generally best assessed with cross-sectional imaging [6].
b. Lateral lumbosacral views and spinopelvic parameters
Statement:
There is no current evidence to support additional lumbar imaging when FAI is suspected in clinical practice, although assessment of spinopelvic parameters and lumbar pathology are increasingly recognized as important in this setting.
Sagittal pelvic kinematics along with spinopelvic (SP) parameters have recently been studied for their effect on hip function, FAI [27, 32, 33], and hip replacement [34]. Variability in sagittal pelvic function may substantially influence impingement phenomena in the native and prosthetic hip [27, 32,33,34], but many of the spine-hip relations are still unexplored.
The consensus panel acknowledges that currently there is not enough evidence to support routine additional lumbar imaging when FAI is suspected. However, when clinically deemed important, imaging evaluation of hip pathology may include a review of SP parameters and assessment for lumbar disease as a hip-pain mimicker, either with a lateral lumbosacral radiograph or by CT/MRI of the entire pelvis with multiplanar reconstructions, instead of imaging only the hip of interest [27, 32, 33].
c. Joint space width and osteoarthritis classification
Statement:
An AP pelvic radiograph with a standardized technique should be preferably used for measuring joint space width and narrowing.
Statement:
Joint space width should be measured where maximal joint space narrowing is observed, preferably at the weight-bearing region of the hip joint.
In hip OA, measurements of joint space width (JSW) and joint space narrowing (JSN) are currently the best ways to assess disease severity and structural progression, respectively [24].
Radiographic measurements of JSN and JSW should be preferably carried out in a standing position (i.e., weight-bearing), which is regarded as more accurate and sensitive for JSN assessment in hips with at least moderate OA [23, 24, 35], although comparable results between standing and supine views have been reported [36]. AP pelvis and hip-centered radiographs offer good and comparable precision for JSW assessment [24, 37].
JSW should be measured in the superior region of the hip joint. It is defined as the distance between the superior contour of the femoral head and the acetabular sourcil (which is the articulating dense line of the superior acetabulum) [36]. The minimum JSW, which is the point of maximal narrowing in the superior region of the joint (Fig. 2), is highly reproducible and has been recommended as the preferred primary structural endpoint in hip OA clinical trials [35].
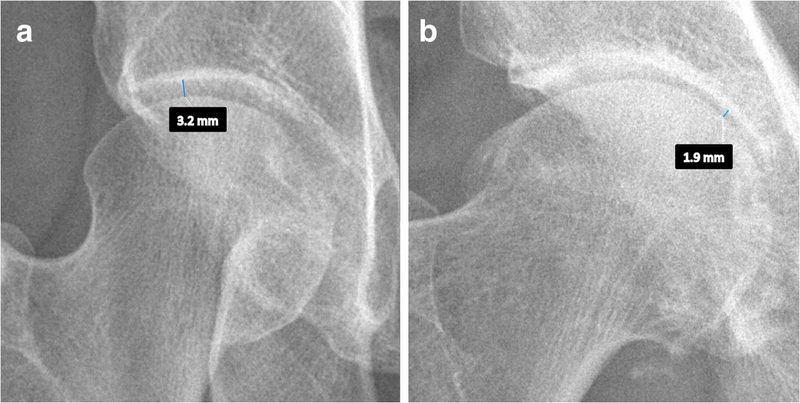
Fig. 2 Examples of measurement of minimum joint space width. This measurement should be carried out in an AP pelvic or hip-centered radiograph, as the inter-bone distance at the point of maximal narrowing in the superior part of the joint space
Alternative projections (e.g., false profile) can evaluate JSW/JSN in locations other than the superior aspect of the joint and, when combined with an AP view, may increase sensitivity to detect structural alterations [38].
Statement:
Tönnis classification represents current clinical and research practice, although evidence supports that the “minimum joint space width” may be preferable compared to the other classification systems.
In the setting of hip OA, quantitative radiographic methods are mostly based on measurements of JSW. Semi-quantitative methods (Supplementary Table 4) identify and categorize different features like JSN, osteophytes, sclerosis, and cyst formation and include the Kellgren and Lawrence (KL) and Tönnis classifications, the Croft score and the Osteoarthritis Research Society International (OARSI) score [39,40,41].
39. Croft P, Cooper C, Wickham C, Coggon D (1990) Defining osteoarthritis of the hip for epidemiologic studies. Am J Epidemiol
132(3):514–522
40. Altman RD, Gold GE (2007) Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthr Cartil 15:A1–A56
41. Kellgren JH, Lawrence JS (1957) Radiological assessment of osteoarthrosis. Ann Rheum Dis 16(4):494–502
The Tönnis classification differentiates 3 grades of hip OA. This system is subjective and has been criticized for its unclear terminology and failure to discriminate overlapping parameters. Accordingly, it has been regarded as a poor method to assess early stages of OA [14, 42], implying that its routine use in surgical decision-making should be abandoned. Nevertheless, it is the most widely used radiographic semi-quantitative classification and outcome predictor in the setting of hip preservation research.
KL grading differentiates 4 grades of OA and has been regarded as the simplest method with excellent inter-reader variability [43]. However, it relies on a mixture of osteoarthritic features and it prejudges a unique sequence of events (osteophytes preceding JSN) which is not the sequence in which events always occur [44]. Additionally, several alternative versions of this scale have been reported [45], with different wording and descriptors, which may lead to inconsistent scoring and unreliable conclusions.
Although KL, OARSI stages, and categorization of JSW have a similar predictive and construct validity, JSW appears to be more reproducible and sensitive to change [43].
MRI
MRI is the gold standard imaging technique for hip and FAI assessment. The role of MRI in suspected FAI is summarized in Fig. 3.
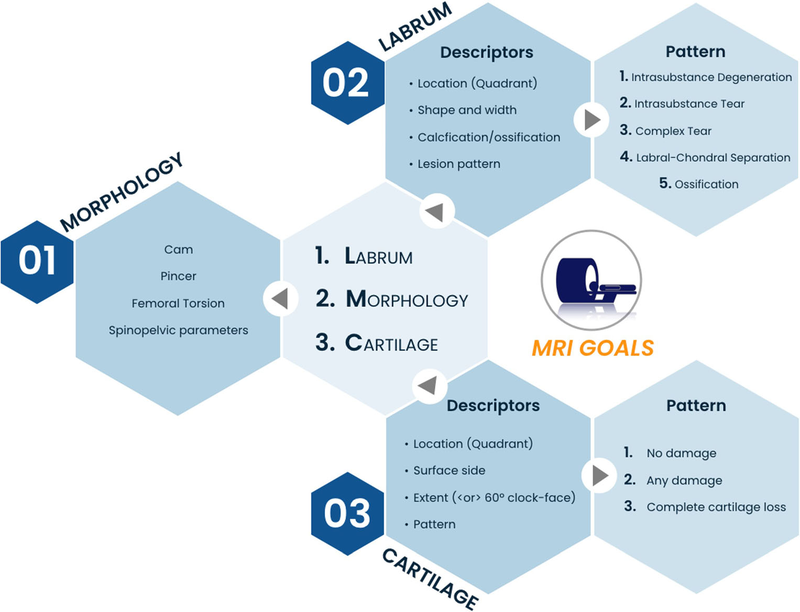
Fig. 3 Imaging goals of MRI in FAI and recommended descriptors of chondrolabral damage. MRI is ideal to characterize bone morphology (particularly Cam morphology), address chondral/labral lesions, and assess differential diagnosis
Technical considerations
Statement:
1.5 T MRI should be considered the minimum recommended field strength for the evaluation of FAI.
Statement :
Generally, direct MR arthrography is superior to non-contrast MRI. Emerging literature suggests that non-contrast 3 T MRI is equivalent to 1.5 T direct arthrography.
Statement :
There is some evidence that direct MR arthrography with hip traction aids in the detection of cartilage delamination. Other applications of traction are still under investigation.
The role of imaging in treatment decision-making is directed mostly to confirmation and characterization of osseous morphologies, determination of chondrolabral damage severity [1, 2, 46] and differential diagnosis assessment (Fig. 1). Radiographs can only indirectly assess articular cartilage damage, by depicting reduced JSW and secondary OA changes, while MRI, MRA, and CTA may demonstrate focal/regional cartilage lesions despite minimal or absent radiographic findings. In cases of high-grade lesions, no significant individual advantage of either of the latter techniques has been demonstrated, as all are able to show severe/extensive chondral damage with relative accuracy, and thus influence the decision of surgical versus non-surgical management [1, 6, 47].
An MRI field strength of 1.5 T should be the minimum to be used in FAI assessment [7, 8]. Unenhanced MRI and direct MRA (dMRA) are the techniques of choice for the detection of hip chondrolabral lesions, although evidence indicates dMRA as the best technique to study intra-articular pathology [7,8,9]. 3-T MRI was reportedly equivalent to 1.5-T dMRA for diagnosing labral tears and cartilage delamination, but superior for acetabular cartilage defects. Additionally, 3-T MRI demonstrated similar sensitivity to 3-T dMRA in the detection of acetabular labral tears, although the latter is more sensitive for the detection of acetabular chondral lesions [7, 8, 48, 49].
Indirect MRA is generally not indicated. Literature is scarce comparing indirect MRA to MRI, although it shows less overall accuracy of indirect MRA when compared to dMRA [7, 8].
There is some evidence that dMRA with hip traction aids in the detection of cartilage delamination both at 1.5 T and 3 T, by uncovering cartilage flaps that are usually less visible on the non-distracted femoral head (Fig. 4). There are no data regarding the use of hip traction during non-arthrographic MRI and only scant data regarding traction with indirect MRA for evaluating labral tears [11, 50]. It is still unclear whether traction at hip MRA should be used routinely and, if so, whether images should be obtained without and with traction, or only with traction.
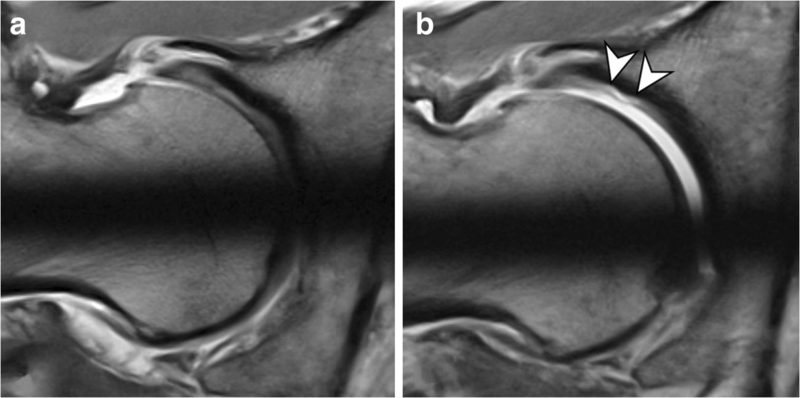
Fig. 4 A 32-year-old man with Cam FAI who was referred for preoperative MR arthrography of the hip for assessment of intra-articular lesions. Radial PD-w images at 3 T were obtained
(a) without traction and (b) with the application of hip traction.
a Without traction, the articular cartilage layers cannot be differentiated and no cartilage damage is evident.
b After joint distraction with 18 kg, contrast agent enters the central joint compartment and undermines the delaminated acetabular cartilage (arrowheads)
Unenhanced CT is not as well established in FAI imaging. Although volumetric CT is excellent at depicting osseous morphologies, assessing osteoarthritic changes [6, 51], and may be used in virtual range of motion 3D simulation studies [6, 51, 52], it is unable to detect chondrolabral changes and it is associated with significant radiation exposure in this frequently young population. While CT is sometimes ordered in conjunction with MRI to assess both bony and soft tissue structures, along with different authors reporting on its use for pre-surgical planning [6, 51, 53], volumetric MRI may be used as a radiation-free all-in-one alternative, helping to reduce costs and time spent in the workup of these patients [6, 51].
Protocol
Statement :
Radial imaging should be routinely obtained in FAI MRI studies.
Statement:
Although radiographs can be used to detect Cam morphology, radial MR imaging is necessary to comprehensively assess the degree and extent of these morphologies by enabling a circumferential evaluation of the femoral head-neck junction.
Statement :
The minimum acceptable number of slices in radial sequences should be 12 slices.
Radial MRI images rotating around the femoral neck axis should be included in all suspected FAI cases, as they allow depiction of FAI morphologies which are typically located anterosuperiorly [6, 27, 54]. Radiographs may help diagnose Cam FAI, although they cannot exclude the presence of Cam morphology and might underestimate its severity [55]. A recent study confirmed this observation and noted the highest correlation between increased alpha angle measured on radial MRI and the Dunn 45° radiographic view, as this view represents the anterosuperior portion of the FHN junction [56]. Thus, either radial proton density–weighted sequences along the axis of the femoral neck (providing higher resolution images) [6, 52] or radial reconstructions from 3D acquisitions [26, 54] should be used, to precisely quantify the asphericity of the FHN junction. Although currently used software-assisted analysis allows for circumferential evaluation of the FHN junction [26, 27, 54], most studies have used at least 12 radial slices [57, 58] (which is easier to apply in clinical practice). Radial sequences seem to have less added value for assessing the acetabulum and/or labrum [59].
Statement:
Femoral torsion should be routinely measured in MRI FAI studies, as this relevant osseous abnormality would be missed otherwise.
There is consistent evidence that abnormal femoral antetorsion is one of the three major osseous abnormalities involved in the development of FAI [25], with a substantial number of patients presenting with abnormally high or low femoral torsion. Several independent authors concluded that femoral torsional profile measurements should be routinely included in the imaging workup of FAI patients [60, 61]. To date, still, no published systematic reviews or meta-analysis are available on this topic.
Statement:
In a young patient with hip pain the MRI protocol should routinely include unilateral small FOV sequences and radial images, as well as femoral torsion assessment and a fluid-sensitive sequence covering the whole pelvis.
While evidence is lacking regarding the ideal hip MRI protocol, sequence details or comparison between protocols, the following sequences are recommended for the assessment of a young patient with hip pain (Fig. 5):
1.Unilateral, small FOV, high-resolution sequences of the symptomatic hip [6, 25, 28, 58];
2.Radial imaging (either through direct acquisition or 3D reformats);
3.Fast axial sequence of the distal knee (femoral condyles) and the proximal femoral neck, to assess femoral torsion;
4.A fluid-sensitive sequence with a large FOV covering the whole pelvis, in the axial or coronal planes, to screen for soft-tissue and bone marrow edema beyond the hip.
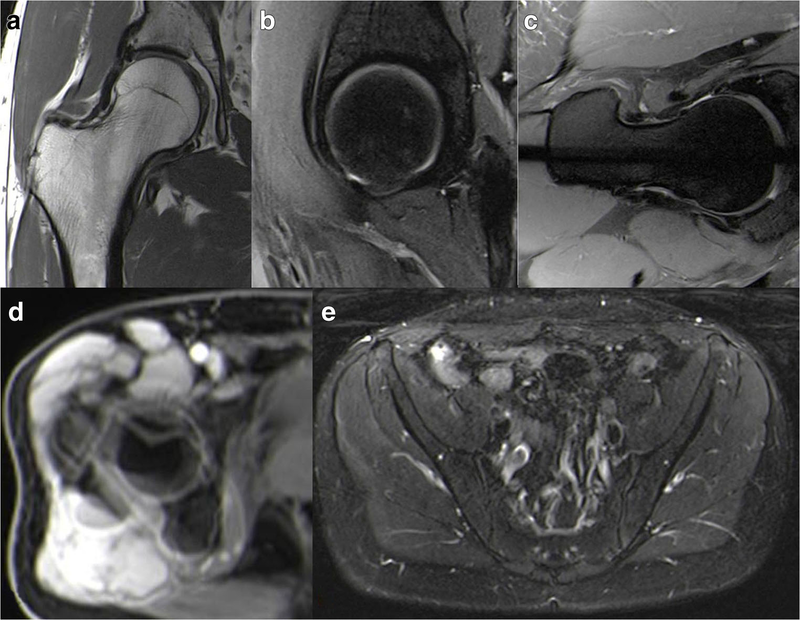
Fig. 5 Sequences that should be incorporated in the proposed MRI protocol for the assessment of suspected FAI. Unilateral hip proton-density 2D sequences, with or without fat-suppression, in the coronal (a), sagittal (b), and axial oblique planes, are used for hip anatomic assessment. Radial imaging, either a 2D proton-density sequence (c) or reformats from a 3D acquisition, is performed to evaluate the morphology of the femoral head-neck junction. For assessing femoral torsion, measurements can be accomplished by superimposing different slices on a single image with post-processing software (d). A 2D axial large-FOV fluid-sensitive sequence of the pelvis (e) is used to screen for other possible causes of the patient’s symptoms
Labrum and cartilage assessment
a.Labrum
Statement :
Although several classifications and grading systems have been proposed for labral lesions, there is no outcome-based evidence to support the use of a specific classification/grading. Description of location, configuration, and extent of labral lesions may be clinically useful.
Statement :
Intrasubstance labral degeneration, labral-chondral separation and to some degree intrasubstance tears are common in asymptomatic individuals without FAI. Labral tears should only be considered relevant in conjunction with patient history and clinical examination.
Several surgical and MRI-based classifications for the description of labrum lesions have been proposed [10, 62]. Currently, no evidence supports the use of a specific description of labral injury based on treatment outcomes. Due to the weak agreement between these classifications, imaging assessment of the acetabular labrum may instead focus on an accurate description, including location, configuration, and extent of labral tears and associated cartilage and osseous changes [63] (Table 3, Fig. 6). Such description can conceptually be applied to any MRI examination with the above-mentioned technique and protocol MR recommendations.
Table 3 Recommended descriptors of labral injury, based on inferential evidence [7, 11, 48]
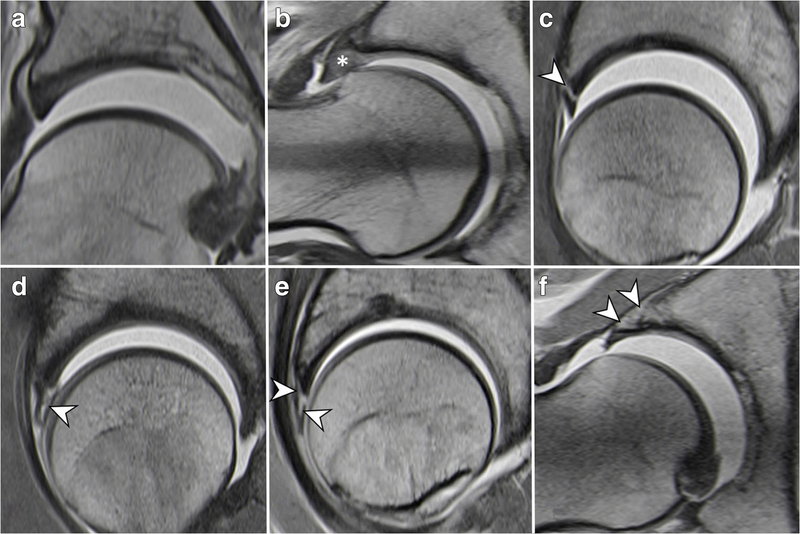
Fig. 6 Classification of labrum damage patterns (traction MRA). Normal labrum (a); Intrasubstance labrum degeneration (asterisk) (b); Labral-chondral separation (= labral detachment) (arrowhead) (c); Intrasubstance labrum tear (arrowhead) (d); Complex labrum tear (labral-chondral separation and intrasubstance labrum tear) (arrowheads) (e); Labral ossification (arrowheads) (f)
Several studies report on intrasubstance labral degeneration, labral-chondral separation, and intrasubstance tears in up to 68% of asymptomatic individuals [64, 65]. Labral tears should only be considered relevant with an adequate patient history and suggestive clinical examination.
b. Cartilage
Statement:
Although available evidence is limited on how cartilage lesions should be reported, description of the extent, location, and pattern/grade is suggested.
Information obtained on hip arthroscopy, during direct observation and probing, is necessarily more comprehensive than the one appreciated on a static examination such as MRI. Consequently, unifying MRI-arthroscopic classifications are deemed to be inconsistent and prone to error. Additionally, there is only outcome-based evidence supporting the description of the extent of cartilage damage by MRI, while only inferential evidence is available for the remaining features. Nevertheless, the description of location, surface, and pattern/grade is recommended by panel members, as it is consistently feasible when using the proposed MRI protocols and technique (Table 4, Fig. 7).
Table 4 Recommended descriptors of cartilage lesions on a hip MRI study
*Recommendations based on outcome evidence
**Recommendations based on inferential evidence
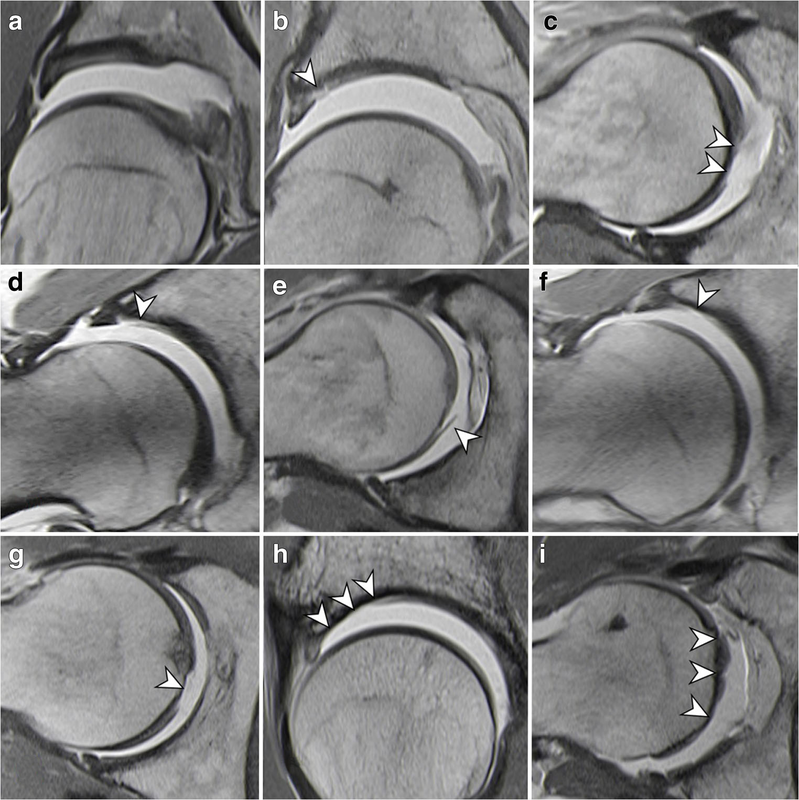
Fig. 7 Classification of femoroacetabular cartilage damage patterns (traction MRA). Grade 1: No damage (normal cartilage thickness) (a). Grade 2: Any cartilage damage, focal acetabular (b) and femoral (c) partial-thickness cartilage lesion (arrowheads). Grade 2: Any cartilage damage, acetabular cartilage delamination involving the chondrolabral junction (d) and femoral cartilage delamination (e) (arrowheads). Grade 3: Complete cartilage loss, focal full-thickness acetabular (f) and femoral (g) cartilage lesion (arrowheads). Grade 3: Complete cartilage loss, diffuse full-thickness acetabular (h) and femoral (i) cartilage lesion (arrowheads)
图7股髋臼软骨损伤类型分类(牵引MRA)。1级:无损伤(软骨厚度正常)(a)。2级:任何软骨损伤,局灶性髋臼(b)和股骨(c)部分软骨病变(箭头)。2级:任何软骨损伤,包括关节关节的髋臼软骨脱层(d)和股骨软骨脱层(e)(箭头)。3级:完全软骨丢失,局灶性全层髋臼(f)和股骨(g)软骨病变(箭头)。3级:完全软骨丢失,弥漫性全层髋臼(h)和股骨(i)软骨病变(箭头)
The extent of cartilage damage evaluated by dMRA is reportedly an independent prognostic factor for long-term outcome of FAI surgery (worse if greater than 60°—see below) [66]. In the presence of extensive cartilage loss, some surgeons may choose not to perform corrective FAI surgery.
The pattern of cartilage lesion can affect surgical planning, including the technique and timing of cartilage repair (e.g., abrasion/microfracture vs. autologous cartilage transplantation) and potential concomitant intra-articular and additional extra-articular osseous procedures.
Location has diagnostic, prognostic and surgical planning implications. Typically located lesions support a FAI mechanism and corresponding treatment, while atypically located lesions may support other etiologies (e.g., trauma, overuse) [58]. Femoral cartilage damage is a poor prognostic factor, frequently indicative of progressive joint degeneration, and may only be accessed via open surgery and not via hip arthroscopy [67, 68]. Additionally, posterior lesions are difficult to access arthroscopically [69].
Statement:
There is only sparse evidence on the prognostic value of intra-articular lesions detected on MRI: femoral and acetabular subchondral cysts, chondral damage exceeding 2 hours on the acetabular clock-face, and central acetabular osteophytes are associated with worse outcomes following FAI surgery.
在MRI上检测到的关节内病变的预后价值只有很少的证据:股骨和髋臼软骨下囊肿、髋臼钟面超过2小时的软骨损伤和髋臼中央骨赘与FAI手术后较差的预后相关。
MRI may aid in treatment decision as it can help to distinguish which patients would benefit from FAI surgery. Although still limited, there is emerging evidence on the prognostic value of intra-articular lesions detected on MRI. Accordingly, femoral and acetabular subchondral cysts are associated with increased rates of clinical failure in the short- and long-term [70, 71]. Cartilage damage exceeding 2 h/60° on the acetabular clock-face and central acetabular osteophytes are poor prognostic factors and are associated with higher rates of clinical failure 10 years after FAI surgery [66].
As most of these lesions might go unrecognized in radiographs, MRI/MRA should be increasingly considered in candidates for hip preserving surgery and included in treatment algorithms [66]. Additionally, a high prevalence of sacroiliac joint abnormalities (25.2%) and a low prevalence (1.8–2.6%) of pubic symphysis abnormalities were reported on imaging in FAI patients. Importantly, these patients may demonstrate significantly inferior clinical outcomes and persistent postoperative pain after FAI treatment [72].
Conclusion
The first international, multidisciplinary Delphi-based consensus regarding imaging of FAI was developed. The panel of experts critically reviewed the roles and limitations of each imaging technique, in light of the available evidence, and highlighted suggested protocols and classifications. In summary, an AP pelvis and a Dunn 45° radiographs are recommended for the initial assessment of FAI. Although radiographs can be used to detect Cam morphology, radial MR imaging is necessary to comprehensively assess the configuration of the FHN junction. MRI is considered the gold standard imaging technique for the detailed evaluation of the hip and FAI patients. The MRI protocol should include unilateral sequences with radial imaging, femoral torsion assessment and a fluid sensitive sequence covering the whole pelvis. Direct MR arthrography is considered superior to non-contrast MRI. The role of other techniques in FAI, such as ultrasound or CT, is still not well defined.
第一个国际性的、多学科的、基于德尔菲的关于FAI成像的共识得到了发展。专家小组根据现有证据严格审查了每种成像技术的作用和局限性,并强调了建议的方案和分类。总之,推荐骨盆AP和Dunn 45°X线片作为FAI的初步评估。虽然X线照片可以用来检测凸轮形态,但径向磁共振成像对于全面评估FHN结的结构是必要的。MRI被认为是详细评估髋关节和FAI患者的金标准成像技术。MRI方案应包括单侧放射成像序列、股骨扭转评估和覆盖整个骨盆的液体敏感序列。直接MR关节造影被认为优于非对比MRI。其他技术在FAI中的作用,如超声或CT,仍然没有很好的定义。
In conclusion, this consensus can help practitioners working with hip-related pain to reduce variability in preoperative intraoperative and postoperative practices, in addition to guide, develop, and implement comprehensive, evidence-based imaging protocols and classifications for future research and clinical management of FAI.
总之,这一共识可以帮助治疗髋关节相关疼痛的从业者减少术前、术中和术后实践的可变性,并为未来FAI的研究和临床管理指导、制定和实施全面的循证成像方案和分类。
Abbreviations
2D:Two dimensional
3D:Three dimensional
AI:Acetabular index
AP:Anteroposterior
CEA:Center-edge angle
CT:Computed tomography
CTA:CT arthrography
dMRA:Direct magnetic resonance arthrography
ESSR:European Society of Musculoskeletal Radiology
FAI:Femoroacetabular impingement
FHN:Femoral head-neck
FOV:Field-of-view
JSN:Joint space narrowing
JSW:Joint space width
KL:Kellgren and Lawrence
L-CEA:Lateral center-edge angle
LOE:Level of evidence
MRA:Magnetic resonance arthrography
MRI:Magnetic resonance imaging
OA:Osteoarthritis
OARSI:Osteoarthritis Research Society International
SP:Spinopelvic

本文为转载文章,如有侵权请联系作者删除。本文仅供健康科普使用,不能做为诊断、治疗的依据,请谨慎参阅





评论